| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-23 18:26:14 UTC |
|---|
| Update Date | 2014-12-24 20:25:58 UTC |
|---|
| Accession Number | T3D3096 |
|---|
| Identification |
|---|
| Common Name | D-Hyoscyamine |
|---|
| Class | Small Molecule |
|---|
| Description | Hyoscyamine is a plant toxin found in certain plants of the Solanaceae family, including henbane (Hyoscyamus niger), mandrake (Mandragora officinarum), jimsonweed (Datura stramonium), and deadly nightshade (Atropa belladonna). It is used as a drug to provide symptomatic relief to various gastrointestinal disorders including spasms, peptic ulcers, irritable bowel syndrome, pancreatitis, colic and cystitis. It has also been used to relieve some heart problems, control some of the symptoms of Parkinson's disease, as well as for control of respiratory secretions in palliative care.(1) |
|---|
| Compound Type | - Amine
- Anti-Arrhythmia Agent
- Antispasmodic
- Drug
- Ester
- Ether
- Muscarinic Antagonist
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
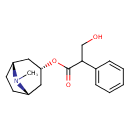
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (-)-Atropine | | (-)-Hyoscyamine | | (S)-(-)-Hyoscyamine | | (S)-(−)-hyoscyamine | | (S)-atropine | | (−)-atropine | | (−)-hyoscyamine | | Acupaz | | Anapaz | | Anaspaz | | Atropen | | Boots Travel Calm | | Buwecon | | Cystospaz | | Cytospaz | | Daturin | | Daturine | | Donnamar | | Duboisine | | Egazil | | Hyoscyamin | | Hyoscyamine | | Hyoscyaminum | | L-Hyoscyamine | | L-Tropine Tropate | | Levbid | | Levsin | | Levsinex | | NuLev | | Symax | | Tropine, (-)-tropate | | Tropine-L-tropate | | [3(S)-Endo]-alpha-(hydroxymethyl)benzeneacetic acid 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester |
|
|---|
| Chemical Formula | C17H23NO3 |
|---|
| Average Molecular Mass | 289.369 g/mol |
|---|
| Monoisotopic Mass | 289.168 g/mol |
|---|
| CAS Registry Number | 13269-35-7 |
|---|
| IUPAC Name | (1R,3S,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl 3-hydroxy-2-phenylpropanoate |
|---|
| Traditional Name | (1R,3S,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl 3-hydroxy-2-phenylpropanoate |
|---|
| SMILES | CN1C2CCC1CC(C2)OC(=O)C(CO)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 |
|---|
| InChI Key | InChIKey=RKUNBYITZUJHSG-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tropane alkaloids. These are organic compounds containing the nitrogenous bicyclic alkaloid parent N-Methyl-8-azabicyclo[3.2.1]octane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Tropane alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tropane alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tropane alkaloid
- Beta-hydroxy acid
- Monocyclic benzene moiety
- Hydroxy acid
- Piperidine
- Benzenoid
- N-alkylpyrrolidine
- Pyrrolidine
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary aliphatic amine
- Tertiary amine
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Organonitrogen compound
- Amine
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organic nitrogen compound
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-5900000000-f705f96b0219dcd47d66 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0006-4900000000-d0c3228da9df55297f7b | 2017-10-06 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-006x-6951100000-dad9042cce0338579e31 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0006-0090000000-b8bc13dbb3c507a0f6b6 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0006-0190000000-c8603eb138fb8f0e3404 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-00dl-3940000000-da1a38da6babdc138c49 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-006x-9700000000-2f9f7ce546a61df93bfa | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0006-9300000000-6a976c404c7b299bbdd6 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0006-9200000000-0eb0ff987d891c85a047 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0006-0090000000-59d025c22f64675dc295 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0006-0090000000-661bc87f4d92ae6353d7 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-00dl-1960000000-dafd68e61cc5909f5dec | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-00dl-7900000000-48d355c9eef2af0979cc | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0006-9300000000-659231292b6eba371158 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , positive | splash10-00di-2900000000-3d5f34ceb343969b3c91 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITTOF , positive | splash10-00fr-0900000000-a216bda74712aba9b341 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-006x-6951100000-dad9042cce0338579e31 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0790000000-b3809a4d9f3d53472626 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1920000000-5cf6a284aa0360bfa6f5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0g02-2900000000-03a359b67b71a8d41b5a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0490000000-42268e6db552a2d85a66 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05g3-1960000000-db1243e11ae5ea609f09 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00bc-5900000000-3453e9ae42b1ccba243c | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (ingestion) (2) ; dermal (2) |
|---|
| Mechanism of Toxicity | Hyoscyamine is an anticholinergic, specifically an antimuscarinic, and works by blocking the action of acetylcholine at parasympathetic sites in smooth muscle, secretory glands, and the central nervous system. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Hyoscyamine is a plant toxin found in certain plants of the Solanaceae family, including henbane (Hyoscyamus niger), mandrake (Mandragora officinarum), jimsonweed (Datura stramonium), and deadly nightshade (Atropa belladonna). It is used as a drug to provide symptomatic relief to various gastrointestinal disorders including spasms, peptic ulcers, irritable bowel syndrome, pancreatitis, colic and cystitis. It has also been used to relieve some heart problems, control some of the symptoms of Parkinson's disease, as well as for control of respiratory secretions in palliative care.(1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Hyoscyamine affects the central nervous system. (1) |
|---|
| Symptoms | Side effects of hyoscyamine include dry mouth and throat, eye pain, blurred vision, restlessness, dizziness, arrythmia, flushing, and faintness. An overdose will cause headache, nausea, vomiting, and central nervous system symptoms including disorientation, hallucinations, euphoria, sexual arousal, short-term memory loss, and possible coma in extreme cases. (1) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00424 |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 637577 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 48882 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | D-Hyoscyamine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Jeffrey Kiel, H. Thomas, Emily Ware, Brady Ware, “Phenolic acid complexes of hyoscyamine and process for preparing the same.” U.S. Patent US20060128637, issued June 15, 2006. |
|---|
| MSDS | Not Available |
|---|
| General References | - Wikipedia. Hyoscyamine. Last Updated 10 June 2009. [Link]
- Wikipedia. Phytotoxin. Last Updated 7 August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | Not Available |
|---|