| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 04:47:20 UTC |
|---|
| Update Date | 2014-12-24 20:26:35 UTC |
|---|
| Accession Number | T3D3960 |
|---|
| Identification |
|---|
| Common Name | Monoisobutyl phthalic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Monoisobutyl phthalic acid is a phthalate metabolite that has been found in human meconium and in semen , saliva , and urine Phthalate esters are a family of multifunctional compounds widely used as plasticizers, solvents, or additives in many diverse products such as poly(vinyl chloride) (PVC) materials, pharmaceuticals and medical devices, pesticides, lubricants, and personal care products. Humans have been exposed to phthalates through the manufacture, ubiquitous use, and disposal of PVC materials and other phthalate-containing products. (1, 2, 3, 1). |
|---|
| Compound Type | - Animal Toxin
- Ester
- Ether
- Food Toxin
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Pesticide
- Plasticizer
- Solvent
|
|---|
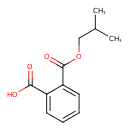
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Isobutyl hydrogen phthalate | | MIBP | | Mono-iso-butyl phthalate | | Monoisobutyl phthalate |
|
|---|
| Chemical Formula | C12H14O4 |
|---|
| Average Molecular Mass | 222.237 g/mol |
|---|
| Monoisotopic Mass | 222.089 g/mol |
|---|
| CAS Registry Number | 30833-53-5 |
|---|
| IUPAC Name | 2-[(2-methylpropoxy)carbonyl]benzoic acid |
|---|
| Traditional Name | MIBP |
|---|
| SMILES | CC(C)COC(=O)C1=CC=CC=C1C(O)=O |
|---|
| InChI Identifier | InChI=1S/C12H14O4/c1-8(2)7-16-12(15)10-6-4-3-5-9(10)11(13)14/h3-6,8H,7H2,1-2H3,(H,13,14) |
|---|
| InChI Key | InChIKey=RZJSUWQGFCHNFS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoic acid esters. These are ester derivatives of benzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Benzoic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzoate ester
- Benzoic acid
- Benzoyl
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-4910000000-ac2484b74eb5cfd5b690 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-6390000000-4ca9de5bf766a991b3dc | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0089-0930000000-d249a5cd21193a8a3cd2 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-0900000000-13d64e3eb0a1e3e235be | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0089-0930000000-3a306e5ce6ca6a0e9127 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0002-0900000000-b4a2e5d94dccaced84c1 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-6690000000-8f32418bd6ccde0549c0 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9720000000-286cdd7561a043eef3d0 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9500000000-6c3aca6817c2db6bb804 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1790000000-2d20ca2ec96d84986321 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0100-2910000000-46c6c191f37b2782021a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-6900000000-0d9a0ca1ebdbc964dac0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4j-0940000000-827565da796a34bbd6b1 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-1900000000-bfe543859b406a2dae92 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2900000000-8cb1cf43ad6057fc27c8 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00b9-0930000000-2a9d225bebcec6f94629 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-6910000000-2ae4c65b2cf89d2dde5a | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9200000000-c321d909ae49bc32f15a | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Phthalates are synthetic industrial compounds capable of disrupting the endocrine system. The phthalates have been the most common chemical compounds with the ability to disrupt the endocrine system. Phthalates, when acting in a critical period of the development of genital tract, lead to disturbances in the androgen-signaling pathway. Fetal testicular dysgenesis syndrome, otherwise known as the ”phthalate syndrome” in rodents, is a consequence of reduced level of fetal testosterone, insulin-like growth factor-3 (IGF-3) and follicule stimulating hormone (FSH). A negative correlation between levels in breast milk and free testosterone of babies was observed, while there was a positive correlation between mono-ethyl phthalate (MEP) and mono-butyl (MBP) with sex hormone bingind globuline (SHBG) and mono-metyl phthalate (MMP) and MEP and MBP with the ratio of lutenzing hormone (LH) and free testosterone. Exposure to phthalates is significantly associated with the duration of pregnancy. According to some studies, the chemical structure of some phthalates and prostaglandin/thromboxane, interleukin-1 connects the phthalates with induction of intrauterine inflammatory processes as well as shortening of pregnancy. (4) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a natural compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB02056 |
|---|
| PubChem Compound ID | 92272 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 83306 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Ke, Changying. Esterification synthesis of diisobutyl phthalate. Huaxue Shijie (1986), 27(10), 440-2. |
|---|
| MSDS | Not Available |
|---|
| General References | - Kato K, Silva MJ, Needham LL, Calafat AM: Quantifying phthalate metabolites in human meconium and semen using automated off-line solid-phase extraction coupled with on-line SPE and isotope-dilution high-performance liquid chromatography--tandem mass spectrometry. Anal Chem. 2006 Sep 15;78(18):6651-5. [16970347 ]
- Silva MJ, Reidy JA, Samandar E, Herbert AR, Needham LL, Calafat AM: Detection of phthalate metabolites in human saliva. Arch Toxicol. 2005 Nov;79(11):647-52. Epub 2005 Jul 2. [15995852 ]
- Silva MJ, Slakman AR, Reidy JA, Preau JL Jr, Herbert AR, Samandar E, Needham LL, Calafat AM: Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Jun 5;805(1):161-7. [15113553 ]
- Bajkin I, Bjelica A, Icin T, Dobric V, Zavisic BK, Stojanoska MM: Effects of phthalic acid esters on fetal health. Med Pregl. 2014 May-Jun;67(5-6):172-5. [25033577 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|