| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-18 21:54:32 UTC |
|---|
| Update Date | 2014-12-24 20:23:06 UTC |
|---|
| Accession Number | T3D1061 |
|---|
| Identification |
|---|
| Common Name | 2-[(2E)-2-(Nitromethylene)hydrazino]naphthalene |
|---|
| Class | Small Molecule |
|---|
| Description | Aromatic heterocycle containing a nitromethylene substituent. Fast acting neurotoxicant, effective both by contact or oral ingestion; they are relatively safe to vertebrates and degrade rapidly in the environment. (1) |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Nitromethylene
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
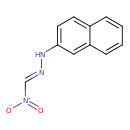
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-[(2e)-2-(nitromethylene)hydrazino]naphthalene | | 2-[(2e)-2-(Nitromethylene)hydrazino]naphthalene |
|
|---|
| Chemical Formula | C11H9N3O2 |
|---|
| Average Molecular Mass | 215.208 g/mol |
|---|
| Monoisotopic Mass | 215.069 g/mol |
|---|
| CAS Registry Number | 302544-16-7 |
|---|
| IUPAC Name | (E)-N'-[(naphthalen-2-yl)amino]-N,N-dioxomethanimidamide |
|---|
| Traditional Name | (E)-N'-(naphthalen-2-ylamino)-N,N-dioxomethanimidamide |
|---|
| SMILES | [O-][N+](=O)\C=N\NC1=CC=C2C=CC=CC2=C1 |
|---|
| InChI Identifier | InChI=1S/C11H9N3O2/c15-14(16)8-12-13-11-6-5-9-3-1-2-4-10(9)7-11/h1-8,13H/b12-8+ |
|---|
| InChI Key | InChIKey=YYXCMCDLDSISNV-XYOKQWHBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthalenes. Naphthalenes are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthalene
- Phenylhydrazine
- C-nitro compound
- Organic nitro compound
- Amidine
- Hydrazone
- Formamidine
- Organic oxoazanium
- Allyl-type 1,3-dipolar organic compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organic oxygen compound
- Organic zwitterion
- Organic nitrogen compound
- Organonitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-8e286582ed9470932c92 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-0890000000-107c4da787c8db568440 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01dr-2910000000-70988e6222e2fcc4a3cd | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-1aec98c75cb25d650b8f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-3190000000-b8fb3e1d3f2fe95dcee5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0w93-9420000000-52ab2b2a35e410dc9852 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Acts as a neurotransmitter mimic , having both excitatory and depressant effects, eventually blocking postsynaptic nicotinic receptors. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Nitromethylenes are used as pesticides. (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Nitromethylenes are neurotoxic. (1) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 9603130 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 7877251 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | 2-[(2E)-2-(Nitromethylene)hydrazino]naphthalene |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|