Methotrexate (T3D2486)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-03 22:10:49 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:35 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2486 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Methotrexate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Methotrexate is only found in individuals that have used or taken this drug. It is an antineoplastic antimetabolite with immunosuppressant properties. It is an inhibitor of tetrahydrofolate dehydrogenase and prevents the formation of tetrahydrofolate, necessary for synthesis of thymidylate, an essential component of DNA. [PubChem]Methotrexate anti-tumor activity is a result of the inhibition of folic acid reductase, leading to inhibition of DNA synthesis and inhibition of cellular replication. The mechanism involved in its activity against rheumatoid arthritis is not known. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
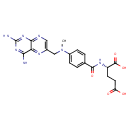
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C20H22N8O5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 454.439 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 454.171 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 59-05-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (2S)-2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}phenyl)formamido]pentanedioic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | methotrexate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [H][C@@](CCC(O)=O)(NC(=O)C1=CC=C(C=C1)N(C)CC1=NC2=C(N)NC(=N)N=C2N=C1)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=FBOZXECLQNJBKD-ZDUSSCGKSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Glutamic acid and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Orange-brown, crystalline powder (5). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Inhalation (2); dermal (2); intravenous (2) Oral absorption is dose dependent in adults and leukemic pediatric patients. In adults, peak serum levels are reached within one to two hours. At doses of 30 mg/m^2 or less, methotrexate is generally well absorbed with a mean bioavailability of 60%. At doses greater than 80 mg/m^2, the absorption of the doses is significantly less due to a saturation effect. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Methotrexate anti-tumor activity is a result of the inhibition of folic acid reductase, leading to inhibition of DNA synthesis and inhibition of cellular replication. The mechanism involved in its activity against rheumatoid arthritis is not known. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | After absorption, methotrexate undergoes hepatic and intracellular metabolism to form methotrexate polyglutamate, metabolites which by hydrolysis may be converted back to methotrexate. Methotrexate polyglutamates inhibit dihydrofolate reductase and thymidylate synthetase. Small amounts of these polyglutamate metabolites may remain in tissues for extended periods; the retention and prolonged action of these active metabolites vary among different cells, tissues, and tumors. In addition, small amounts of methotrexate polyglutamate may be converted to 7-hydroxymethotrexate; accumulation of this metabolite may become substantial following administration of high doses of methotrexate, since the aqueous solubility of 7-hydroxymethotrexate is threefold to fivefold lower than that of the parent compound. Following oral administration of methotrexate, the drug also is partially metabolized by the intestinal flora. Renal excretion is the primary route of elimination, and is dependent upon dosage and route of administration (6). Route of Elimination: Renal excretion is the primary route of elimination and is dependent upon dosage and route of administration. IV administration, 80% to 90% of the administered dose is excreted unchanged in the urine within 24 hours. There is limited biliary excretion amounting to 10% or less of the administered dose. Half Life: Low doses (less than 30 mg/m^2): 3 to 10 hours; High doses: 8 to 15 hours. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 43 mg/kg (Oral, Rat) (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (10) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Methotrexate is indicated in the treatment of gestational choriocarcinoma, chorioadenoma destruens and hydatidiform mole. In acute lymphocytic leukemia, methotrexate is indicated in the prophylaxis of meningeal leukemia and is used in maintenance therapy in combination with other chemotherapeutic agents. Methotrexate is also indicated in the treatment of meningeal leukemia. Methotrexate is used alone or in combination with other anticancer agents in the treatment of breast cancer, epidermoid cancers of the head and neck, advanced mycosis fungoides (cutaneous T cell lymphoma), and lung cancer, particularly squamous cell and small cell types. Methotrexate is also used in combination with other chemotherapeutic agents in the treatment of advanced stage non-Hodgkin's lymphomas. Methotrexate is indicated in the symptomatic control of severe, recalcitrant, disabling psoriasis. Methotrexate is indicated in the management of selected adults with severe, active rheumatoid arthritis (ACR criteria), or children with active polyarticular-course juvenile rheumatoid arthritis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | A small percentage of patients develop hepatitis, and there is an increased risk of pulmonary fibrosis where dry cough can be an important sign. The higher doses of methotrexate often used in cancer chemotherapy can cause toxic effects to the rapidly-dividing cells of bone marrow and gastrointestinal mucosa. The resulting myelosuppression and mucositis are often prevented (termed Leucovorin "rescue"- as this is the folic acid based drug used) (11). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of overdose include bone marrow suppression and gastrointestinal toxicity. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Administer charcoal as a slurry. Consider gastric lavage after ingestion of a potentially life-threatening amount of poison if it can be performed soon after ingestion (generally within 1 hour). Protect airway by placement in Trendelenburg and left lateral decubitus position or by endotracheal intubation. Control any seizures first. Glucarpidase has been used intravenously in combination with thymidine and leucovorin to treat methotrexate toxicity. (7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00563 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB14703 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 126941 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL34259 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 112728 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C01937 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 6837 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | CPD-6041 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | D008727 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Methotrexate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | MTX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Methotrexate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Nadph binding
- Specific Function:
- Key enzyme in folate metabolism. Contributes to the de novo mitochondrial thymidylate biosynthesis pathway. Catalyzes an essential reaction for de novo glycine and purine synthesis, and for DNA precursor synthesis. Binds its own mRNA and that of DHFRL1.
- Gene Name:
- DHFR
- Uniprot ID:
- P00374
- Molecular Weight:
- 21452.61 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0 uM | Not Available | BindingDB 18050 |
| Inhibitory | 0.00000519 uM | Not Available | BindingDB 18050 |
| Inhibitory | 0.000038 uM | Not Available | BindingDB 18050 |
| Inhibitory | 0.00011 uM | Not Available | BindingDB 18050 |
| Inhibitory | 0.000179 uM | Not Available | BindingDB 18050 |
| Inhibitory | 4.42 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0006 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0007 uM | Not Available | BindingDB 18050 |
| IC50 | 0.00082 uM | Not Available | BindingDB 18050 |
| IC50 | 0.001 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0012 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0017 uM | Not Available | BindingDB 18050 |
| IC50 | 0.002 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0021 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0027 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0033 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0038 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0039 uM | Not Available | BindingDB 18050 |
| IC50 | 0.004 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0043 uM | Not Available | BindingDB 18050 |
| IC50 | 0.006 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0079 uM | Not Available | BindingDB 18050 |
| IC50 | 0.009 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0096 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0112 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0138 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0145 uM | Not Available | BindingDB 18050 |
| IC50 | 0.02 uM | Not Available | BindingDB 18050 |
| IC50 | 0.022 uM | Not Available | BindingDB 18050 |
| IC50 | 0.024 uM | Not Available | BindingDB 18050 |
| IC50 | 0.0243 uM | Not Available | BindingDB 18050 |
| IC50 | 0.025 uM | Not Available | BindingDB 18050 |
| IC50 | 0.026 uM | Not Available | BindingDB 18050 |
| IC50 | 0.03 uM | Not Available | BindingDB 18050 |
| IC50 | 0.03294 uM | Not Available | BindingDB 18050 |
| IC50 | 0.035 uM | Not Available | BindingDB 18050 |
| IC50 | 0.038 uM | Not Available | BindingDB 18050 |
| IC50 | 0.04 uM | Not Available | BindingDB 18050 |
| IC50 | 0.044 uM | Not Available | BindingDB 18050 |
| IC50 | 0.066 uM | Not Available | BindingDB 18050 |
| IC50 | 0.08 uM | Not Available | BindingDB 18050 |
| IC50 | 0.72 uM | Not Available | BindingDB 18050 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Totani K, Matsuo I, Ihara Y, Ito Y: High-mannose-type glycan modifications of dihydrofolate reductase using glycan-methotrexate conjugates. Bioorg Med Chem. 2006 Aug 1;14(15):5220-9. Epub 2006 May 2. [16647263 ]
- Uga H, Kuramori C, Ohta A, Tsuboi Y, Tanaka H, Hatakeyama M, Yamaguchi Y, Takahashi T, Kizaki M, Handa H: A new mechanism of methotrexate action revealed by target screening with affinity beads. Mol Pharmacol. 2006 Nov;70(5):1832-9. Epub 2006 Aug 25. [16936229 ]
- Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AA, Abdel-Hamide SG, Youssef KM, Al-Obaid AM, El-Subbagh HI: Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem. 2006 Dec 15;14(24):8608-21. Epub 2006 Sep 12. [16971132 ]
- Bennett B, Langan P, Coates L, Mustyakimov M, Schoenborn B, Howell EE, Dealwis C: Neutron diffraction studies of Escherichia coli dihydrofolate reductase complexed with methotrexate. Proc Natl Acad Sci U S A. 2006 Dec 5;103(49):18493-8. Epub 2006 Nov 27. [17130456 ]
- Assaraf YG: Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007 Mar;26(1):153-81. [17333344 ]
- Hart BP, Haile WH, Licato NJ, Bolanowska WE, McGuire JJ, Coward JK: Synthesis and biological activity of folic acid and methotrexate analogues containing L-threo-(2S,4S)-4-fluoroglutamic acid and DL-3,3-difluoroglutamic acid. J Med Chem. 1996 Jan 5;39(1):56-65. [8568827 ]
- Gangjee A, Devraj R, McGuire JJ, Kisliuk RL: Effect of bridge region variation on antifolate and antitumor activity of classical 5-substituted 2,4-diaminofuro[2,3-d]pyrimidines. J Med Chem. 1995 Sep 15;38(19):3798-805. [7562910 ]
- Tsukamoto T, Haile WH, McGuire JJ, Coward JK: Synthesis and biological evaluation of N alpha-(4-amino-4-deoxy-10-methylpteroyl)-DL-4,4-difluoroornithine. J Med Chem. 1996 Jun 21;39(13):2536-40. [8691451 ]
- Thurmond J, Butchbach ME, Palomo M, Pease B, Rao M, Bedell L, Keyvan M, Pai G, Mishra R, Haraldsson M, Andresson T, Bragason G, Thosteinsdottir M, Bjornsson JM, Coovert DD, Burghes AH, Gurney ME, Singh J: Synthesis and biological evaluation of novel 2,4-diaminoquinazoline derivatives as SMN2 promoter activators for the potential treatment of spinal muscular atrophy. J Med Chem. 2008 Feb 14;51(3):449-69. doi: 10.1021/jm061475p. Epub 2008 Jan 19. [18205293 ]
- Nair MG, Salter DC, Kisliuk RL, Gaumont Y, North G, Sirotnak FM: Folate analogues. 21. Synthesis and antifolate and antitumor activities of N10-(cyanomethyl)-5,8-dideazafolic acid. J Med Chem. 1983 Apr;26(4):605-7. [6403710 ]
- Taylor EC, Harrington PJ, Fletcher SR, Beardsley GP, Moran RG: Synthesis of the antileukemic agents 5,10-dideazaaminopterin and 5,10-dideaza-5,6,7,8-tetrahydroaminopterin. J Med Chem. 1985 Jul;28(7):914-21. [4009615 ]
- Gangjee A, Namjoshi OA, Raghavan S, Queener SF, Kisliuk RL, Cody V: Design, synthesis, and molecular modeling of novel pyrido[2,3-d]pyrimidine analogues as antifolates; application of Buchwald-Hartwig aminations of heterocycles. J Med Chem. 2013 Jun 13;56(11):4422-41. doi: 10.1021/jm400086g. Epub 2013 May 21. [23627352 ]
- Graffner-Nordberg M, Marelius J, Ohlsson S, Persson A, Swedberg G, Andersson P, Andersson SE, Aqvist J, Hallberg A: Computational predictions of binding affinities to dihydrofolate reductase: synthesis and biological evaluation of methotrexate analogues. J Med Chem. 2000 Oct 19;43(21):3852-61. [11052790 ]
- Cao SL, Han Y, Yuan CZ, Wang Y, Xiahou ZK, Liao J, Gao RT, Mao BB, Zhao BL, Li ZF, Xu X: Synthesis and antiproliferative activity of 4-substituted-piperazine-1-carbodithioate derivatives of 2,4-diaminoquinazoline. Eur J Med Chem. 2013 Jun;64:401-9. doi: 10.1016/j.ejmech.2013.04.017. Epub 2013 Apr 15. [23665106 ]
- Rosowsky A, Forsch RA, Reich VE, Freisheim JH, Moran RG: Side chain modified 5-deazafolate and 5-deazatetrahydrofolate analogues as mammalian folylpolyglutamate synthetase and glycinamide ribonucleotide formyltransferase inhibitors: synthesis and in vitro biological evaluation. J Med Chem. 1992 May 1;35(9):1578-88. [1578484 ]
- Nair MG, Nanavati NT, Nair IG, Kisliuk RL, Gaumont Y, Hsiao MC, Kalman TI: Folate analogues. 26. Syntheses and antifolate activity of 10-substituted derivatives of 5,8-dideazafolic acid and of the poly-gamma-glutamyl metabolites of N10-propargyl-5,8-dideazafolic acid (PDDF). J Med Chem. 1986 Sep;29(9):1754-60. [3091832 ]
- Rosowsky A, Forsch RA, Yu CS, Lazarus H, Beardsley GP: Methotrexate analogues. 21. Divergent influence of alkyl chain length on the dihydrofolate reductase affinity and cytotoxicity of methotrexate monoesters. J Med Chem. 1984 May;27(5):605-9. [6585550 ]
- Hynes JB, Patil SA, Tomazic A, Kumar A, Pathak A, Tan XH, Li XQ, Ratnam M, Delcamp TJ, Freisheim JH: Inhibition of murine thymidylate synthase and human dihydrofolate reductase by 5,8-dideaza analogues of folic acid and aminopterin. J Med Chem. 1988 Feb;31(2):449-54. [3339615 ]
- Abraham A, McGuire JJ, Galivan J, Nimec Z, Kisliuk RL, Gaumont Y, Nair MG: Folate analogues. 34. Synthesis and antitumor activity of non-polyglutamylatable inhibitors of dihydrofolate reductase. J Med Chem. 1991 Jan;34(1):222-7. [1992121 ]
- Gangjee A, Devraj R, McGuire JJ, Kisliuk RL, Queener SF, Barrows LR: Classical and nonclassical furo[2,3-d]pyrimidines as novel antifolates: synthesis and biological activities. J Med Chem. 1994 Apr 15;37(8):1169-76. [8164259 ]
- Hynes JB, Patil SA, Hagan RL, Cole A, Kohler W, Freisheim JH: Comparison of the biological effects of selected 5,8-dideazafolate analogues with their 2-desamino counterparts. J Med Chem. 1989 Apr;32(4):852-6. [2704031 ]
- Patil SA, Shane B, Freisheim JH, Singh SK, Hynes JB: Inhibition of mammalian folylpolyglutamate synthetase and human dihydrofolate reductase by 5,8-dideaza analogues of folic acid and aminopterin bearing a terminal L-ornithine. J Med Chem. 1989 Jul;32(7):1559-65. [2738891 ]
- Gangjee A, Yu J, McGuire JJ, Cody V, Galitsky N, Kisliuk RL, Queener SF: Design, synthesis, and X-ray crystal structure of a potent dual inhibitor of thymidylate synthase and dihydrofolate reductase as an antitumor agent. J Med Chem. 2000 Oct 19;43(21):3837-51. [11052789 ]
- Nair MG, Chen SY, Kisliuk RL, Gaumont Y, Strumpf D: Folate analogues altered in the C9-N10 bridge region. 16. Synthesis and antifolate activity of 11-thiohomoaminopterin. J Med Chem. 1980 Aug;23(8):899-903. [6772788 ]
- Gangjee A, Jain HD, McGuire JJ, Kisliuk RL: Benzoyl ring halogenated classical 2-amino-6-methyl-3,4-dihydro-4-oxo-5-substituted thiobenzoyl-7H-pyrrolo[2,3-d]pyrimidine antifolates as inhibitors of thymidylate synthase and as antitumor agents. J Med Chem. 2004 Dec 30;47(27):6730-9. [15615522 ]
- Santos MA, Enyedy EA, Nuti E, Rossello A, Krupenko NI, Krupenko SA: Methotrexate gamma-hydroxamate derivatives as potential dual target antitumor drugs. Bioorg Med Chem. 2007 Feb 1;15(3):1266-74. Epub 2006 Nov 14. [17127067 ]
- Rosowsky A, Bader H, Wright JE, Keyomarsi K, Matherly LH: Synthesis and biological activity of N omega-hemiphthaloyl-alpha,omega- diaminoalkanoic acid analogues of aminopterin and 3',5-dichloroaminopterin. J Med Chem. 1994 Jul 8;37(14):2167-74. [8035423 ]
- Rosowsky A, Forsch RA, Freisheim JH, Moran RG: The 2-desamino and 2-desamino-2-methyl analogues of aminopterin do not inhibit dihydrofolate reductase but are potently toxic to tumor cells in culture. J Med Chem. 1989 Mar;32(3):517-20. [2918496 ]
- Reynolds RC, Campbell SR, Fairchild RG, Kisliuk RL, Micca PL, Queener SF, Riordan JM, Sedwick WD, Waud WR, Leung AK, Dixon RW, Suling WJ, Borhani DW: Novel boron-containing, nonclassical antifolates: synthesis and preliminary biological and structural evaluation. J Med Chem. 2007 Jul 12;50(14):3283-9. Epub 2007 Jun 15. [17569517 ]
- Gangjee A, Li W, Yang J, Kisliuk RL: Design, synthesis, and biological evaluation of classical and nonclassical 2-amino-4-oxo-5-substituted-6-methylpyrrolo[3,2-d]pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors. J Med Chem. 2008 Jan 10;51(1):68-76. Epub 2007 Dec 12. [18072727 ]
- Gangjee A, Qiu Y, Li W, Kisliuk RL: Potent dual thymidylate synthase and dihydrofolate reductase inhibitors: classical and nonclassical 2-amino-4-oxo-5-arylthio-substituted-6-methylthieno[2,3-d]pyrimidine antifolates. J Med Chem. 2008 Sep 25;51(18):5789-97. doi: 10.1021/jm8006933. [18800768 ]
- Gangjee A, Li W, Kisliuk RL, Cody V, Pace J, Piraino J, Makin J: Design, synthesis, and X-ray crystal structure of classical and nonclassical 2-amino-4-oxo-5-substituted-6-ethylthieno[2,3-d]pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors and as potential antitumor agents. J Med Chem. 2009 Aug 13;52(15):4892-902. doi: 10.1021/jm900490a. [19719239 ]
- Gangjee A, Zhao Y, Ihnat MA, Thorpe JE, Bailey-Downs LC, Kisliuk RL: Novel tricyclic indeno[2,1-d]pyrimidines with dual antiangiogenic and cytotoxic activities as potent antitumor agents. Bioorg Med Chem. 2012 Jul 15;20(14):4217-25. doi: 10.1016/j.bmc.2012.05.068. Epub 2012 Jun 6. [22739090 ]
- Gangjee A, Zeng Y, McGuire JJ, Kisliuk RL: Effect of C9-methyl substitution and C8-C9 conformational restriction on antifolate and antitumor activity of classical 5-substituted 2,4-diaminofuro[2,3-d]pyrimidines. J Med Chem. 2000 Aug 10;43(16):3125-33. [10956221 ]
- Gangjee A, Zeng Y, McGuire JJ, Kisliuk RL: Synthesis of N-[4-[1-ethyl-2-(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid as an antifolate. J Med Chem. 2002 Apr 25;45(9):1942-8. [11960504 ]
- Gangjee A, Zeng Y, McGuire JJ, Kisliuk RL: Synthesis of classical and nonclassical, partially restricted, linear, tricyclic 5-deaza antifolates. J Med Chem. 2002 Nov 7;45(23):5173-81. [12408727 ]
- Gangjee A, Yu J, Kisliuk RL, Haile WH, Sobrero G, McGuire JJ: Design, synthesis, and biological activities of classical N-[4-[2-(2-amino-4-ethylpyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-l-glutamic acid and its 6-methyl derivative as potential dual inhibitors of thymidylate synthase and dihydrofolate reductase and as potential antitumor agents. J Med Chem. 2003 Feb 13;46(4):591-600. [12570380 ]
- Gangjee A, Zeng Y, McGuire JJ, Mehraein F, Kisliuk RL: Synthesis of classical, three-carbon-bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates. J Med Chem. 2004 Dec 30;47(27):6893-901. [15615538 ]
- Gangjee A, Zeng Y, McGuire JJ, Kisliuk RL: Synthesis of classical, four-carbon bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates. J Med Chem. 2005 Aug 11;48(16):5329-36. [16078850 ]
- Gangjee A, Lin X, Kisliuk RL, McGuire JJ: Synthesis of N-{4-[(2,4-diamino-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]benz oyl}-L-glutamic acid and N-{4-[(2-amino-4-oxo-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]be nzoyl}-L-glutamic acid as dual inhibitors of dihydrofolate reductase and thymidylate synthase and as potential antitumor agents. J Med Chem. 2005 Nov 17;48(23):7215-22. [16279780 ]
- Gangjee A, Zeng Y, Talreja T, McGuire JJ, Kisliuk RL, Queener SF: Design and synthesis of classical and nonclassical 6-arylthio-2,4-diamino-5-ethylpyrrolo[2,3-d]pyrimidines as antifolates. J Med Chem. 2007 Jun 28;50(13):3046-53. Epub 2007 Jun 7. [17552508 ]
- Gangjee A, Jain HD, Queener SF, Kisliuk RL: The effect of 5-alkyl modification on the biological activity of pyrrolo[2,3-d]pyrimidine containing classical and nonclassical antifolates as inhibitors of dihydrofolate reductase and as antitumor and/or antiopportunistic infection agents. J Med Chem. 2008 Aug 14;51(15):4589-600. doi: 10.1021/jm800244v. Epub 2008 Jul 8. [18605720 ]
- Gangjee A, Jain HD, Phan J, Guo X, Queener SF, Kisliuk RL: 2,4-Diamino-5-methyl-6-substituted arylthio-furo[2,3-d]pyrimidines as novel classical and nonclassical antifolates as potential dual thymidylate synthase and dihydrofolate reductase inhibitors. Bioorg Med Chem. 2010 Jan 15;18(2):953-61. doi: 10.1016/j.bmc.2009.11.029. Epub 2009 Dec 26. [20056546 ]
- Gangjee A, Zaware N, Raghavan S, Ihnat M, Shenoy S, Kisliuk RL: Single agents with designed combination chemotherapy potential: synthesis and evaluation of substituted pyrimido[4,5-b]indoles as receptor tyrosine kinase and thymidylate synthase inhibitors and as antitumor agents. J Med Chem. 2010 Feb 25;53(4):1563-78. doi: 10.1021/jm9011142. [20092323 ]
- Zhang X, Zhou X, Kisliuk RL, Piraino J, Cody V, Gangjee A: Design, synthesis, biological evaluation and X-ray crystal structure of novel classical 6,5,6-tricyclic benzo[4,5]thieno[2,3-d]pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors. Bioorg Med Chem. 2011 Jun 1;19(11):3585-94. doi: 10.1016/j.bmc.2011.03.067. Epub 2011 Apr 9. [21550809 ]
- Rosowsky A, Forsch RA, Moran RG, Freisheim JH: Synthesis and biological activity of the 2-desamino and 2-desamino-2-methyl analogues of aminopterin and methotrexate. J Med Chem. 1991 Jan;34(1):227-34. [1992122 ]
- Rosowsky A, Bader H, Freisheim JH: Synthesis and biological activity of methotrexate analogues with two acid groups and a hydrophobic aromatic ring in the side chain. J Med Chem. 1991 Feb;34(2):574-9. [1995880 ]
- Barrow EW, Bourne PC, Barrow WW: Functional cloning of Bacillus anthracis dihydrofolate reductase and confirmation of natural resistance to trimethoprim. Antimicrob Agents Chemother. 2004 Dec;48(12):4643-9. [15561838 ]
- Rosowsky A, Bader H, Freisheim JH: Analogues of methotrexate and aminopterin with gamma-methylene and gamma-cyano substitution of the glutamate side chain: synthesis and in vitro biological activity. J Med Chem. 1991 Jan;34(1):203-8. [1992118 ]
- Piper JR, Montgomery JA: Convenient synthesis of 10-deazaaminopterin via a pteridine ylide. J Med Chem. 1980 Mar;23(3):320-1. [7365749 ]
- DeGraw JI, Christie PH, Kisliuk RL, Gaumont Y, Sirotnak FM: Synthesis and antifolate properties of 9-alkyl-10-deazaminopterins. J Med Chem. 1990 Jan;33(1):212-5. [2296020 ]
- Zhang Z, Wu J, Ran F, Guo Y, Tian R, Zhou S, Wang X, Liu Z, Zhang L, Cui J, Liu J: Novel 8-deaza-5,6,7,8-tetrahydroaminopterin derivatives as dihydrofolate inhibitor: design, synthesis and antifolate activity. Eur J Med Chem. 2009 Feb;44(2):764-71. doi: 10.1016/j.ejmech.2008.04.017. Epub 2008 May 4. [18555562 ]
- Gangjee A, Vasudevan A, Queener SF, Kisliuk RL: 2,4-diamino-5-deaza-6-substituted pyrido[2,3-d]pyrimidine antifolates as potent and selective nonclassical inhibitors of dihydrofolate reductases. J Med Chem. 1996 Mar 29;39(7):1438-46. [8691474 ]
- Gangjee A, Vidwans A, Elzein E, McGuire JJ, Queener SF, Kisliuk RL: Synthesis, antifolate, and antitumor activities of classical and nonclassical 2-amino-4-oxo-5-substituted-pyrrolo[2,3-d]pyrimidines. J Med Chem. 2001 Jun 7;44(12):1993-2003. [11384244 ]
- Al-Omary FA, Abou-Zeid LA, Nagi MN, Habib el-SE, Abdel-Aziz AA, El-Azab AS, Abdel-Hamide SG, Al-Omar MA, Al-Obaid AM, El-Subbagh HI: Non-classical antifolates. Part 2: synthesis, biological evaluation, and molecular modeling study of some new 2,6-substituted-quinazolin-4-ones. Bioorg Med Chem. 2010 Apr 15;18(8):2849-63. doi: 10.1016/j.bmc.2010.03.019. Epub 2010 Mar 12. [20350811 ]
- Tsukamoto T, Kitazume T, McGuire JJ, Coward JK: Synthesis and biological evaluation of DL-4,4-difluoroglutamic acid and DL-gamma,gamma-difluoromethotrexate. J Med Chem. 1996 Jan 5;39(1):66-72. [8568828 ]
- Rosowsky A, Mota CE, Queener SF, Waltham M, Ercikan-Abali E, Bertino JR: 2,4-Diamino-5-substituted-quinazolines as inhibitors of a human dihydrofolate reductase with a site-directed mutation at position 22 and of the dihydrofolate reductases from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1995 Mar 3;38(5):745-52. [7877140 ]
- Corona P, Gibellini F, Cavalli A, Saxena P, Carta A, Loriga M, Luciani R, Paglietti G, Guerrieri D, Nerini E, Gupta S, Hannaert V, Michels PA, Ferrari S, Costi PM: Structure-based selectivity optimization of piperidine-pteridine derivatives as potent Leishmania pteridine reductase inhibitors. J Med Chem. 2012 Oct 11;55(19):8318-29. doi: 10.1021/jm300563f. Epub 2012 Sep 19. [22946585 ]
- Rosowsky A, Wright JE, Vaidya CM, Bader H, Forsch RA, Mota CE, Pardo J, Chen CS, Chen YN: Synthesis and potent antifolate activity and cytotoxicity of B-ring deaza analogues of the nonpolyglutamatable dihydrofolate reductase inhibitor Nalpha-(4-amino-4-deoxypteroyl)-Ndelta-hemiphthaloyl- L-ornithine (PT523). J Med Chem. 1998 Dec 17;41(26):5310-9. [9857098 ]
- Zuccotto F, Brun R, Gonzalez Pacanowska D, Ruiz Perez LM, Gilbert IH: The structure-based design and synthesis of selective inhibitors of Trypanosoma cruzi dihydrofolate reductase. Bioorg Med Chem Lett. 1999 May 17;9(10):1463-8. [10360757 ]
- Corona P, Loriga M, Costi MP, Ferrari S, Paglietti G: Synthesis of N-(5,7-diamino-3-phenyl-quinoxalin-2-yl)-3,4,5-substituted anilines and N-[4[(5,7-diamino-3-phenylquinoxalin-2-yl)amino]benzoyl]-l-glutamic acid diethyl ester: evaluation of in vitro anti-cancer and anti-folate activities. Eur J Med Chem. 2008 Jan;43(1):189-203. Epub 2007 Apr 18. [17532099 ]
- General Function:
- Thymidylate synthase activity
- Specific Function:
- Contributes to the de novo mitochondrial thymidylate biosynthesis pathway.
- Gene Name:
- TYMS
- Uniprot ID:
- P04818
- Molecular Weight:
- 35715.65 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.6 uM | Not Available | BindingDB 18050 |
| IC50 | 29 uM | Not Available | BindingDB 18050 |
| IC50 | 36 uM | Not Available | BindingDB 18050 |
| IC50 | 40 uM | Not Available | BindingDB 18050 |
| IC50 | 75 uM | Not Available | BindingDB 18050 |
| IC50 | 86 uM | Not Available | BindingDB 18050 |
| IC50 | 100 uM | Not Available | BindingDB 18050 |
| IC50 | 120 uM | Not Available | BindingDB 18050 |
| IC50 | 170 uM | Not Available | BindingDB 18050 |
| IC50 | 190 uM | Not Available | BindingDB 18050 |
| IC50 | >18 uM | Not Available | BindingDB 18050 |
References
- Gangjee A, Lin X, Kisliuk RL, McGuire JJ: Synthesis of N-{4-[(2,4-diamino-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]benz oyl}-L-glutamic acid and N-{4-[(2-amino-4-oxo-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]be nzoyl}-L-glutamic acid as dual inhibitors of dihydrofolate reductase and thymidylate synthase and as potential antitumor agents. J Med Chem. 2005 Nov 17;48(23):7215-22. [16279780 ]
- Gangjee A, Jain HD, Queener SF, Kisliuk RL: The effect of 5-alkyl modification on the biological activity of pyrrolo[2,3-d]pyrimidine containing classical and nonclassical antifolates as inhibitors of dihydrofolate reductase and as antitumor and/or antiopportunistic infection agents. J Med Chem. 2008 Aug 14;51(15):4589-600. doi: 10.1021/jm800244v. Epub 2008 Jul 8. [18605720 ]
- Gangjee A, Jain HD, Phan J, Guo X, Queener SF, Kisliuk RL: 2,4-Diamino-5-methyl-6-substituted arylthio-furo[2,3-d]pyrimidines as novel classical and nonclassical antifolates as potential dual thymidylate synthase and dihydrofolate reductase inhibitors. Bioorg Med Chem. 2010 Jan 15;18(2):953-61. doi: 10.1016/j.bmc.2009.11.029. Epub 2009 Dec 26. [20056546 ]
- Gangjee A, Vidwans A, Elzein E, McGuire JJ, Queener SF, Kisliuk RL: Synthesis, antifolate, and antitumor activities of classical and nonclassical 2-amino-4-oxo-5-substituted-pyrrolo[2,3-d]pyrimidines. J Med Chem. 2001 Jun 7;44(12):1993-2003. [11384244 ]
- Nair MG, Chen SY, Kisliuk RL, Gaumont Y, Strumpf D: Folate analogues altered in the C9-N10 bridge region. 16. Synthesis and antifolate activity of 11-thiohomoaminopterin. J Med Chem. 1980 Aug;23(8):899-903. [6772788 ]
- Piper JR, McCaleb GS, Montgomery JA, Kisliuk RL, Gaumont Y, Sirotnak FM: 10-Propargylaminopterin and alkyl homologues of methotrexate as inhibitors of folate metabolism. J Med Chem. 1982 Jul;25(7):877-80. [7108907 ]
- DeGraw JI, Colwell WT, Brown VH, Sato M, Kisliuk RL, Gaumont Y, Thorndike J, Sirotnak FM: Synthesis and biological evaluation of 8-deazahomofolic acid and its tetrahydro derivative. J Med Chem. 1988 Jan;31(1):150-3. [3121855 ]
- DeGraw JI, Colwell WT, Kisliuk RL, Gaumont Y, Sirotnak FM: Synthesis and antifolate properties of 5,10-methylenetetrahydro-8,10-dideazaminopterin. J Med Chem. 1986 Sep;29(9):1786-9. [3091834 ]
- Nair MG, Nanavati NT, Nair IG, Kisliuk RL, Gaumont Y, Hsiao MC, Kalman TI: Folate analogues. 26. Syntheses and antifolate activity of 10-substituted derivatives of 5,8-dideazafolic acid and of the poly-gamma-glutamyl metabolites of N10-propargyl-5,8-dideazafolic acid (PDDF). J Med Chem. 1986 Sep;29(9):1754-60. [3091832 ]
- Gangjee A, Devraj R, McGuire JJ, Kisliuk RL: Effect of bridge region variation on antifolate and antitumor activity of classical 5-substituted 2,4-diaminofuro[2,3-d]pyrimidines. J Med Chem. 1995 Sep 15;38(19):3798-805. [7562910 ]
- Gangjee A, Devraj R, McGuire JJ, Kisliuk RL, Queener SF, Barrows LR: Classical and nonclassical furo[2,3-d]pyrimidines as novel antifolates: synthesis and biological activities. J Med Chem. 1994 Apr 15;37(8):1169-76. [8164259 ]
- Taylor EC, Harrington PJ, Fletcher SR, Beardsley GP, Moran RG: Synthesis of the antileukemic agents 5,10-dideazaaminopterin and 5,10-dideaza-5,6,7,8-tetrahydroaminopterin. J Med Chem. 1985 Jul;28(7):914-21. [4009615 ]
- Zhang X, Zhou X, Kisliuk RL, Piraino J, Cody V, Gangjee A: Design, synthesis, biological evaluation and X-ray crystal structure of novel classical 6,5,6-tricyclic benzo[4,5]thieno[2,3-d]pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors. Bioorg Med Chem. 2011 Jun 1;19(11):3585-94. doi: 10.1016/j.bmc.2011.03.067. Epub 2011 Apr 9. [21550809 ]
- Corona P, Gibellini F, Cavalli A, Saxena P, Carta A, Loriga M, Luciani R, Paglietti G, Guerrieri D, Nerini E, Gupta S, Hannaert V, Michels PA, Ferrari S, Costi PM: Structure-based selectivity optimization of piperidine-pteridine derivatives as potent Leishmania pteridine reductase inhibitors. J Med Chem. 2012 Oct 11;55(19):8318-29. doi: 10.1021/jm300563f. Epub 2012 Sep 19. [22946585 ]
- General Function:
- Toxic substance binding
- Specific Function:
- Serum albumin, the main protein of plasma, has a good binding capacity for water, Ca(2+), Na(+), K(+), fatty acids, hormones, bilirubin and drugs. Its main function is the regulation of the colloidal osmotic pressure of blood. Major zinc transporter in plasma, typically binds about 80% of all plasma zinc.
- Gene Name:
- ALB
- Uniprot ID:
- P02768
- Molecular Weight:
- 69365.94 Da
References
- Bolling C, Graefe T, Lubbing C, Jankevicius F, Uktveris S, Cesas A, Meyer-Moldenhauer WH, Starkmann H, Weigel M, Burk K, Hanauske AR: Phase II study of MTX-HSA in combination with cisplatin as first line treatment in patients with advanced or metastatic transitional cell carcinoma. Invest New Drugs. 2006 Nov;24(6):521-7. [16699974 ]
- Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB: Removal of methotrexate by peritoneal dialysis and hemodialysis in a single patient with end-stage renal disease. Am J Med Sci. 2006 Sep;332(3):156-8. [16969149 ]
- Xie WJ, Feng YP, Cao SL, Zhao YF: [Study of the interaction between methotrexate and bovine serum albumin by spectrometry]. Guang Pu Xue Yu Guang Pu Fen Xi. 2006 Oct;26(10):1876-9. [17205742 ]
- Kratz F, Abu Ajaj K, Warnecke A: Anticancer carrier-linked prodrugs in clinical trials. Expert Opin Investig Drugs. 2007 Jul;16(7):1037-58. [17594188 ]
- Warnecke A, Fichtner I, Sass G, Kratz F: Synthesis, cleavage profile, and antitumor efficacy of an albumin-binding prodrug of methotrexate that is cleaved by plasmin and cathepsin B. Arch Pharm (Weinheim). 2007 Aug;340(8):389-95. [17628030 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Bifunctional enzyme that catalyzes 2 steps in purine biosynthesis.Promotes insulin receptor/INSR autophosphorylation and is involved in INSR internalization (PubMed:25687571).
- Gene Name:
- ATIC
- Uniprot ID:
- P31939
- Molecular Weight:
- 64615.255 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >20 uM | Not Available | BindingDB 18050 |
References
- DeGraw JI, Colwell WT, Brown VH, Sato M, Kisliuk RL, Gaumont Y, Thorndike J, Sirotnak FM: Synthesis and biological evaluation of 8-deazahomofolic acid and its tetrahydro derivative. J Med Chem. 1988 Jan;31(1):150-3. [3121855 ]
- General Function:
- Reduced folate carrier activity
- Specific Function:
- Transporter for the intake of folate. Uptake of folate in human placental choriocarcinoma cells occurs by a novel mechanism called potocytosis which functionally couples three components, namely the folate receptor, the folate transporter, and a V-type H(+)-pump.
- Gene Name:
- SLC19A1
- Uniprot ID:
- P41440
- Molecular Weight:
- 64867.62 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 3.5 uM | Not Available | BindingDB 18050 |
References
- Bavetsias V, Marriott JH, Melin C, Kimbell R, Matusiak ZS, Boyle FT, Jackman AL: Design and synthesis of Cyclopenta[g]quinazoline-based antifolates as inhibitors of thymidylate synthase and potential antitumor agents(,). J Med Chem. 2000 May 18;43(10):1910-26. [10821704 ]
- General Function:
- Tetrahydrofolylpolyglutamate synthase activity
- Specific Function:
- Catalyzes conversion of folates to polyglutamate derivatives allowing concentration of folate compounds in the cell and the intracellular retention of these cofactors, which are important substrates for most of the folate-dependent enzymes that are involved in one-carbon transfer reactions involved in purine, pyrimidine and amino acid synthesis. Unsubstituted reduced folates are the preferred substrates. Metabolizes methotrexate (MTX) to polyglutamates.
- Gene Name:
- FPGS
- Uniprot ID:
- Q05932
- Molecular Weight:
- 64608.53 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 3.2 uM | Not Available | BindingDB 18050 |
References
- Mao Z, Pan J, Kalman TI: Design and synthesis of histidine analogues of folic acid and methotrexate as potential folylpolyglutamate synthetase inhibitors. J Med Chem. 1996 Oct 11;39(21):4340-4. [8863812 ]
- General Function:
- Transcription coactivator activity
- Specific Function:
- Nuclear receptor coactivator that directly binds nuclear receptors and stimulates the transcriptional activities in a hormone-dependent fashion. Involved in the coactivation of different nuclear receptors, such as for steroids (PGR, GR and ER), retinoids (RXRs), thyroid hormone (TRs) and prostanoids (PPARs). Also involved in coactivation mediated by STAT3, STAT5A, STAT5B and STAT6 transcription factors. Displays histone acetyltransferase activity toward H3 and H4; the relevance of such activity remains however unclear. Plays a central role in creating multisubunit coactivator complexes that act via remodeling of chromatin, and possibly acts by participating in both chromatin remodeling and recruitment of general transcription factors. Required with NCOA2 to control energy balance between white and brown adipose tissues. Required for mediating steroid hormone response. Isoform 2 has a higher thyroid hormone-dependent transactivation activity than isoform 1 and isoform 3.
- Gene Name:
- NCOA1
- Uniprot ID:
- Q15788
- Molecular Weight:
- 156755.44 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >35.879 uM | Not Available | BindingDB 18050 |
References
- Liu T, Lin Y, Wen X, Jorissen RN, Gilson MK: BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007 Jan;35(Database issue):D198-201. Epub 2006 Dec 1. [17145705 ]
- General Function:
- Transcriptional activator activity, rna polymerase ii core promoter proximal region sequence-specific binding
- Specific Function:
- Nuclear receptor coactivator that directly binds nuclear receptors and stimulates the transcriptional activities in a hormone-dependent fashion. Plays a central role in creating a multisubunit coactivator complex, which probably acts via remodeling of chromatin. Involved in the coactivation of different nuclear receptors, such as for steroids (GR and ER), retinoids (RARs and RXRs), thyroid hormone (TRs), vitamin D3 (VDR) and prostanoids (PPARs). Displays histone acetyltransferase activity. Also involved in the coactivation of the NF-kappa-B pathway via its interaction with the NFKB1 subunit.
- Gene Name:
- NCOA3
- Uniprot ID:
- Q9Y6Q9
- Molecular Weight:
- 155292.535 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >35.879 uM | Not Available | BindingDB 18050 |
References
- Liu T, Lin Y, Wen X, Jorissen RN, Gilson MK: BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007 Jan;35(Database issue):D198-201. Epub 2006 Dec 1. [17145705 ]
- General Function:
- Protein-arginine deiminase activity
- Specific Function:
- Catalyzes the citrullination/deimination of arginine residues of proteins such as histones, thereby playing a key role in histone code and regulation of stem cell maintenance. Citrullinates histone H1 at 'Arg-54' (to form H1R54ci), histone H3 at 'Arg-2', 'Arg-8', 'Arg-17' and/or 'Arg-26' (to form H3R2ci, H3R8ci, H3R17ci, H3R26ci, respectively) and histone H4 at 'Arg-3' (to form H4R3ci). Acts as a key regulator of stem cell maintenance by mediating citrullination of histone H1: citrullination of 'Arg-54' of histone H1 (H1R54ci) results in H1 displacement from chromatin and global chromatin decondensation, thereby promoting pluripotency and stem cell maintenance. Promotes profound chromatin decondensation during the innate immune response to infection in neutrophils by mediating formation of H1R54ci. Citrullination of histone H3 prevents their methylation by CARM1 and HRMT1L2/PRMT1 and represses transcription. Citrullinates EP300/P300 at 'Arg-2142', which favors its interaction with NCOA2/GRIP1.
- Gene Name:
- PADI4
- Uniprot ID:
- Q9UM07
- Molecular Weight:
- 74078.65 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >10000 uM | Not Available | BindingDB 18050 |
References
- Knuckley B, Luo Y, Thompson PR: Profiling Protein Arginine Deiminase 4 (PAD4): a novel screen to identify PAD4 inhibitors. Bioorg Med Chem. 2008 Jan 15;16(2):739-45. Epub 2007 Oct 13. [17964793 ]
- General Function:
- Phosphoribosylglycinamide formyltransferase activity
- Specific Function:
- Not Available
- Gene Name:
- GART
- Uniprot ID:
- P22102
- Molecular Weight:
- 107766.295 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >20 uM | Not Available | BindingDB 18050 |
References
- DeGraw JI, Colwell WT, Brown VH, Sato M, Kisliuk RL, Gaumont Y, Thorndike J, Sirotnak FM: Synthesis and biological evaluation of 8-deazahomofolic acid and its tetrahydro derivative. J Med Chem. 1988 Jan;31(1):150-3. [3121855 ]
- General Function:
- Not Available
- Specific Function:
- Not Available
- Gene Name:
- TP53
- Uniprot ID:
- P04637
- Molecular Weight:
- 43652.79 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| AC50 | 0.39 uM | APR_p53Act_72h_up | Apredica |
References
- Sipes NS, Martin MT, Kothiya P, Reif DM, Judson RS, Richard AM, Houck KA, Dix DJ, Kavlock RJ, Knudsen TB: Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem Res Toxicol. 2013 Jun 17;26(6):878-95. doi: 10.1021/tx400021f. Epub 2013 May 16. [23611293 ]