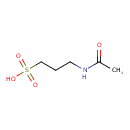
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007o-9100000000-8420bb56903c56b30a73 | 2017-09-01 | View Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-1900000000-095fc64fb4490a8d6b4d | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-c177d5ee7162b0b8756d | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-40eaa1bb480a68330940 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-1900000000-35bfe6cae36755d1cf1c | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0059-9500000000-d366cda3213379d7501b | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9000000000-4374601f59e1dc8df0eb | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9000000000-011f30e0257faffd4553 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-c179316d784a91f87c59 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-8cf123c7cf7dddcf6838 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-1900000000-84a7d1cc6ca5022d4da7 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0059-9500000000-4503d9afc6bab741521b | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9000000000-8c2ba21c044eeada7839 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-004i-9000000000-dd14c920c5211780d172 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-1900000000-dac38f625c8648b643c4 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0006-0900000000-3684e08456a971400c3d | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0006-0900000000-6137fe4463b3675f55eb | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0900000000-58f776a52000101b8fb7 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0006-0900000000-d8de420d40b45a34ea95 | 2017-09-14 | View Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-001l-0900000000-1bb28abe8034966ad829 | 2017-09-14 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0019-0900000000-343cd651472c88796a61 | 2016-08-03 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05g0-5900000000-0c71e48c69c18769563d | 2016-08-03 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9300000000-181e815cd85a7505ce4a | 2016-08-03 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-4900000000-1f6b18b2f30371ea12ba | 2016-08-03 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9500000000-50bd4d569514037edb1b | 2016-08-03 | View Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-ec0629ff72c5e58a0413 | 2016-08-03 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |