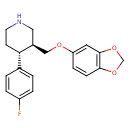
Paroxetine (T3D2868)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:27:29 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:52 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2868 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Paroxetine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Paroxetine hydrochloride and paroxetine mesylate belong to a class of antidepressant agents known as selective serotonin-reuptake inhibitors (SSRIs). Despite distinct structural differences between compounds in this class, SSRIs possess similar pharmacological activity. As with other antidepressant agents, several weeks of therapy may be required before a clinical effect is seen. SSRIs are potent inhibitors of neuronal serotonin reuptake. They have little to no effect on norepinephrine or dopamine reuptake and do not antagonize α- or β-adrenergic, dopamine D2 or histamine H1 receptors. During acute use, SSRIs block serotonin reuptake and increase serotonin stimulation of somatodendritic 5-HT1A and terminal autoreceptors. Chronic use leads to desensitization of somatodendritic 5-HT1A and terminal autoreceptors. The overall clinical effect of increased mood and decreased anxiety is thought to be due to adaptive changes in neuronal function that leads to enhanced serotonergic neurotransmission. Side effects include dry mouth, nausea, dizziness, drowsiness, sexual dysfunction and headache (see Toxicity section below for a complete listing of side effects). Side effects generally occur during the first two weeks of therapy and are usually less severe and frequent than those observed with tricyclic antidepressants. Paroxetine hydrochloride and mesylate are considered therapeutic alternatives rather than generic equivalents by the US Food and Drug Administration (FDA); both agents contain the same active moiety (i.e. paroxetine), but are formulated as different salt forms. Clinical studies establishing the efficacy of paroxetine in various conditions were performed using paroxetine hydrochloride. Since both agents contain the same active moiety, the clinical efficacy of both agents is thought to be similar. Paroxetine may be used to treat major depressive disorder (MDD), panic disorder with or without agoraphobia, obsessive-compulsive disorder (OCD), social anxiety disorder (social phobia), generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD) and premenstrual dysphoric disorder (PMDD). Paroxetine has the most evidence supporting its use for anxiety-related disorders of the SSRIs. It has the greatest anticholinergic activity of the agents in this class and compared to other SSRIs, paroxetine may cause greater weight gain, sexual dysfunction, sedation and constipation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C19H20FNO3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 329.365 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 329.143 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 61869-08-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (3S,4R)-3-[(2H-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | paroxetine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [H][C@@]1(COC2=CC3=C(OCO3)C=C2)CNCC[C@@]1([H])C1=CC=C(F)C=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=AHOUBRCZNHFOSL-YOEHRIQHSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as phenylpiperidines. Phenylpiperidines are compounds containing a phenylpiperidine skeleton, which consists of a piperidine bound to a phenyl group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Piperidines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phenylpiperidines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Phenylpiperidines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Paroxetine hydrochloride is slowly, but completely absorbed following oral administration. Paroxetine mesylate salt is also completely absorbed after oral dosing. The oral bioavailability appears to be low due to extensive first-pass metabolism. Paroxetine hydrochloride oral tablets and suspension are reportedly bioequivalent. Absorption of either salt form is not substantially affected by food. Peak concentrations of Brisbelle (mesylate salt) were reached at 6 hours (3 to 8 hours range). Steady state Cmax was 13.10 ng/mL. The steady state AUC (0-last) was 237 hr*ng/mL. Paroxetine mesylate generally follows non-linear pharmacokinetics because CYP2D6, the enzyme that is part responisible for paroxetine metabolism, is readily saturable. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Paroxetine is a potent and highly selective inhibitor of neuronal serotonin reuptake. Paroxetine likely inhibits the reuptake of serotonin at the neuronal membrane, enhances serotonergic neurotransmission by reducing turnover of the neurotransmitter, therefore it prolongs its activity at synaptic receptor sites and potentiates 5-HT in the CNS; paroxetine is more potent than both sertraline and fluoxetine in its ability to inhibit 5-HT reuptake. Compared to the tricyclic antidepressants, SSRIs have dramatically decreased binding to histamine, acetylcholine, and norepinephrine receptors. The mechanism of action for the treatment of vasomotor symptoms is unknown. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Paroxetine is extensively metabolized after oral administration, likely in the liver. The main metabolites are polar and conjugated products of oxidation and methylation, which are readily eliminated by the body. The predominant metabolites are glucuronic acid and sulfate conjugates. Paroxetine metabolites do not possess significant pharmacologic activity (less than 2% that of parent compound). Paroxetine is metabolized by cytochrome P450 (CYP) 2D6. Enzyme saturation appears to account for the nonlinear pharmacokinetics observed with increasing dose and duration of therapy. Route of Elimination: Approximately 64% of a 30 mg oral solution of paroxetine was excreted in the urine with 2% as the parent compound and 62% as metabolites. Approximately 36% of the dose was excreted in the feces (via bile), mostly as metabolites and less than 1% as parent compound. Half Life: 21-24 hours | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 500mg/kg (Oral, Mouse) (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Labeled indications include: major depressive disorder (MDD), panic disorder with or without agoraphobia, obsessive-compulsive disorder (OCD), social anxiety disorder (social phobia), generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), and premenstrual dysphoric disorder (PMDD). Unlabeled indications include: eating disorders, impulse control disorders, vasomotor symptoms of menopause, obsessive-compulsive disorder (OCD) in children, and mild dementia-associated agitation in nonpsychotic individuals. Brisdelle, which consists of paroxetine mesylate is indicated for the treatment of moderate to severe vasomotor symptoms (like hot flashes) associated with menopause. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Coma, dizziness, drowsiness, facial flushing, nausea, sweating, tremor, vomiting | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Treatment should consist of those general measures employed in the management of overdosage with any drugs effective in the treatment of major depressive disorder. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion, or in symptomatic patients. Activated charcoal should be administered. Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are unlikely to be of benefit. No specific antidotes for paroxetine are known. (8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00715 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB14853 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 43815 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL490 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 39888 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C07415 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 7936 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Paroxetine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Paroxetine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Charles M. Zepp, Yun Gao, Donald L. Heefner, “Method of preparing optically pure precursors of paroxetine.” U.S. Patent US5258517, issued January, 1976. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Serotonin:sodium symporter activity
- Specific Function:
- Serotonin transporter whose primary function in the central nervous system involves the regulation of serotonergic signaling via transport of serotonin molecules from the synaptic cleft back into the pre-synaptic terminal for re-utilization. Plays a key role in mediating regulation of the availability of serotonin to other receptors of serotonergic systems. Terminates the action of serotonin and recycles it in a sodium-dependent manner.
- Gene Name:
- SLC6A4
- Uniprot ID:
- P31645
- Molecular Weight:
- 70324.165 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.00004 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.00006 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.00009 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.0001 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.00013 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.00034 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.00038 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.00042 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.00083 uM | Not Available | BindingDB 22416 |
| IC50 | 0.0002 uM | Not Available | BindingDB 22416 |
| IC50 | 0.002 uM | Not Available | BindingDB 22416 |
| Dissociation | 0.00013 uM | Not Available | BindingDB 22416 |
References
- Scholze P, Zwach J, Kattinger A, Pifl C, Singer EA, Sitte HH: Transporter-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J Pharmacol Exp Ther. 2000 Jun;293(3):870-8. [10869387 ]
- Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, Davis S, Kirshner MA, Houck PR, Stack JA, Reynolds CF, Kupfer DJ: Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology. 2000 Nov;23(5):587-90. [11027924 ]
- Preuss UW, Soyka M, Bahlmann M, Wenzel K, Behrens S, de Jonge S, Kruger M, Bondy B: Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationships to impulsivity. Psychiatry Res. 2000 Sep 25;96(1):51-61. [10980326 ]
- Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR: The effects of methamphetamine on serotonin transporter activity: role of dopamine and hyperthermia. J Neurochem. 2000 Oct;75(4):1608-17. [10987842 ]
- Wihlback AC, Sundstrom-Poromaa I, Allard P, Mjorndal T, Spigset O, Backstrom T: Influence of postmenopausal hormone replacement therapy on platelet serotonin uptake site and serotonin 2A receptor binding. Obstet Gynecol. 2001 Sep;98(3):450-7. [11530128 ]
- Carlier PR, Lo MM, Lo PC, Richelson E, Tatsumi M, Reynolds IJ, Sharma TA: Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett. 1998 Mar 3;8(5):487-92. [9871604 ]
- Wu YJ, He H, Bertekap R, Westphal R, Lelas S, Newton A, Wallace T, Taber M, Davis C, Macor JE, Bronson J: Discovery of disubstituted piperidines and homopiperidines as potent dual NK1 receptor antagonists-serotonin reuptake transporter inhibitors for the treatment of depression. Bioorg Med Chem. 2013 Apr 15;21(8):2217-28. doi: 10.1016/j.bmc.2013.02.010. Epub 2013 Feb 19. [23477943 ]
- McComas CC, Vu AT, Mahaney PE, Cohn ST, Fensome A, Marella MA, Nogle L, Trybulski EJ, Ye F, Zhang P, Alfinito P, Bray J, Johnston G, Koury E, Deecher DC: Synthesis and activity of 1-(3-amino-1-phenylpropyl)indolin-2-ones: a new class of selective norepinephrine reuptake inhibitors. Bioorg Med Chem Lett. 2008 Sep 15;18(18):4929-31. doi: 10.1016/j.bmcl.2008.08.060. Epub 2008 Aug 22. [18771916 ]
- Mattson RJ, Catt JD, Denhart DJ, Deskus JA, Ditta JL, Higgins MA, Marcin LR, Sloan CP, Beno BR, Gao Q, Cunningham MA, Mattson GK, Molski TF, Taber MT, Lodge NJ: Conformationally restricted homotryptamines. 2. Indole cyclopropylmethylamines as selective serotonin reuptake inhibitors. J Med Chem. 2005 Sep 22;48(19):6023-34. [16162005 ]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB: Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997 Dec;283(3):1305-22. [9400006 ]
- Gelmi ML, Caputo F, Clerici F, Pellegrino S, Giannaccini G, Betti L, Fabbrini L, Schmid L, Palego L, Lucacchini A: 1-Aminocyclopentane-1,2,4-tricarboxylic acids screening on glutamatergic and serotonergic systems. Bioorg Med Chem. 2007 Dec 15;15(24):7581-9. Epub 2007 Sep 12. [17900912 ]
- Owens JM, Knight DL, Nemeroff CB: [Second generation SSRIS: human monoamine transporter binding profile of escitalopram and R-fluoxetine]. Encephale. 2002 Jul-Aug;28(4):350-5. [12232544 ]
- Tatsumi M, Groshan K, Blakely RD, Richelson E: Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997 Dec 11;340(2-3):249-58. [9537821 ]
- Ben-Daniel R, Deuther-Conrad W, Scheunemann M, Steinbach J, Brust P, Mishani E: Carbon-11 labeled indolylpropylamine analog as a new potential PET agent for imaging of the serotonin transporter. Bioorg Med Chem. 2008 Jun 15;16(12):6364-70. doi: 10.1016/j.bmc.2008.05.006. Epub 2008 May 7. [18487050 ]
- Funke U, Fischer S, Hiller A, Scheunemann M, Deuther-Conrad W, Brust P, Steinbach J: 3-(4-(6-Fluoroalkoxy-3,4-dihydroisoquinoline-2(1H)-yl)cyclohexyl)-1H-indole-5-car bonitriles for SERT imaging: chemical synthesis, evaluation in vitro and radiofluorination. Bioorg Med Chem Lett. 2008 Aug 15;18(16):4727-30. doi: 10.1016/j.bmcl.2008.06.077. Epub 2008 Jun 28. [18644726 ]
- Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, Newman AH, Blakely RD: Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2006 Jan 27;281(4):2012-23. Epub 2005 Nov 3. [16272152 ]
- General Function:
- Norepinephrine:sodium symporter activity
- Specific Function:
- Amine transporter. Terminates the action of noradrenaline by its high affinity sodium-dependent reuptake into presynaptic terminals.
- Gene Name:
- SLC6A2
- Uniprot ID:
- P23975
- Molecular Weight:
- 69331.42 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.04 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.045 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.085 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.09 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.156 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.328 uM | Not Available | BindingDB 22416 |
| IC50 | 0.1 uM | Not Available | BindingDB 22416 |
| Dissociation | 0.04 uM | Not Available | BindingDB 22416 |
References
- Rubin RT: Paroxetine binding to the rat norepinephrine transporter in vivo. Biol Psychiatry. 2000 Nov 1;48(9):954-6. [11203183 ]
- Gilmor ML, Owens MJ, Nemeroff CB: Inhibition of norepinephrine uptake in patients with major depression treated with paroxetine. Am J Psychiatry. 2002 Oct;159(10):1702-10. [12359676 ]
- Nemeroff CB, Owens MJ: Neuropharmacology of paroxetine. Psychopharmacol Bull. 2003 Spring;37 Suppl 1:8-18. [14566196 ]
- Carlier PR, Lo MM, Lo PC, Richelson E, Tatsumi M, Reynolds IJ, Sharma TA: Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett. 1998 Mar 3;8(5):487-92. [9871604 ]
- McComas CC, Vu AT, Mahaney PE, Cohn ST, Fensome A, Marella MA, Nogle L, Trybulski EJ, Ye F, Zhang P, Alfinito P, Bray J, Johnston G, Koury E, Deecher DC: Synthesis and activity of 1-(3-amino-1-phenylpropyl)indolin-2-ones: a new class of selective norepinephrine reuptake inhibitors. Bioorg Med Chem Lett. 2008 Sep 15;18(18):4929-31. doi: 10.1016/j.bmcl.2008.08.060. Epub 2008 Aug 22. [18771916 ]
- Tatsumi M, Groshan K, Blakely RD, Richelson E: Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997 Dec 11;340(2-3):249-58. [9537821 ]
- Owens JM, Knight DL, Nemeroff CB: [Second generation SSRIS: human monoamine transporter binding profile of escitalopram and R-fluoxetine]. Encephale. 2002 Jul-Aug;28(4):350-5. [12232544 ]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB: Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997 Dec;283(3):1305-22. [9400006 ]
- Mattson RJ, Catt JD, Denhart DJ, Deskus JA, Ditta JL, Higgins MA, Marcin LR, Sloan CP, Beno BR, Gao Q, Cunningham MA, Mattson GK, Molski TF, Taber MT, Lodge NJ: Conformationally restricted homotryptamines. 2. Indole cyclopropylmethylamines as selective serotonin reuptake inhibitors. J Med Chem. 2005 Sep 22;48(19):6023-34. [16162005 ]
- General Function:
- Virus receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including mescaline, psilocybin, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates phospholipase C and a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and promotes the release of Ca(2+) ions from intracellular stores. Affects neural activity, perception, cognition and mood. Plays a role in the regulation of behavior, including responses to anxiogenic situations and psychoactive substances. Plays a role in intestinal smooth muscle contraction, and may play a role in arterial vasoconstriction.(Microbial infection) Acts as a receptor for human JC polyomavirus/JCPyV.
- Gene Name:
- HTR2A
- Uniprot ID:
- P28223
- Molecular Weight:
- 52602.58 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 22416 |
References
- Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, Wilson AA, Rafi-Tari S, Mayberg HS, Kennedy SH: The effect of paroxetine on 5-HT(2A) receptors in depression: an [(18)F]setoperone PET imaging study. Am J Psychiatry. 2001 Jan;158(1):78-85. [11136637 ]
- Zanardi R, Artigas F, Moresco R, Colombo C, Messa C, Gobbo C, Smeraldi E, Fazio F: Increased 5-hydroxytryptamine-2 receptor binding in the frontal cortex of depressed patients responding to paroxetine treatment: a positron emission tomography scan study. J Clin Psychopharmacol. 2001 Feb;21(1):53-8. [11199948 ]
- Bixo M, Allard P, Backstrom T, Mjorndal T, Nyberg S, Spigset O, Sundstrom-Poromaa I: Binding of [3H]paroxetine to serotonin uptake sites and of [3H]lysergic acid diethylamide to 5-HT2A receptors in platelets from women with premenstrual dysphoric disorder during gonadotropin releasing hormone treatment. Psychoneuroendocrinology. 2001 Aug;26(6):551-64. [11403977 ]
- Kojima H, Terao T, Iwakawa M, Soya A, Inoue N, Shiraishi Y, Son Y, Soeda S, Ueda N, Yoshimura R, Nakamura J: Paroxetine as a 5-HT neuroendocrine probe. Psychopharmacology (Berl). 2003 Apr;167(1):97-102. Epub 2003 Feb 25. [12601506 ]
- Messa C, Colombo C, Moresco RM, Gobbo C, Galli L, Lucignani G, Gilardi MC, Rizzo G, Smeraldi E, Zanardi R, Artigas F, Fazio F: 5-HT(2A) receptor binding is reduced in drug-naive and unchanged in SSRI-responder depressed patients compared to healthy controls: a PET study. Psychopharmacology (Berl). 2003 Apr;167(1):72-8. Epub 2003 Mar 11. [12632246 ]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM: RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997 Apr-May;36(4-5):621-9. [9225287 ]
- General Function:
- Monoamine transmembrane transporter activity
- Specific Function:
- Amine transporter. Terminates the action of dopamine by its high affinity sodium-dependent reuptake into presynaptic terminals.
- Gene Name:
- SLC6A3
- Uniprot ID:
- Q01959
- Molecular Weight:
- 68494.255 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.268 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.4 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.49 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.963 uM | Not Available | BindingDB 22416 |
| Dissociation | 0.49 uM | Not Available | BindingDB 22416 |
References
- Carlier PR, Lo MM, Lo PC, Richelson E, Tatsumi M, Reynolds IJ, Sharma TA: Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett. 1998 Mar 3;8(5):487-92. [9871604 ]
- Owens JM, Knight DL, Nemeroff CB: [Second generation SSRIS: human monoamine transporter binding profile of escitalopram and R-fluoxetine]. Encephale. 2002 Jul-Aug;28(4):350-5. [12232544 ]
- Mattson RJ, Catt JD, Denhart DJ, Deskus JA, Ditta JL, Higgins MA, Marcin LR, Sloan CP, Beno BR, Gao Q, Cunningham MA, Mattson GK, Molski TF, Taber MT, Lodge NJ: Conformationally restricted homotryptamines. 2. Indole cyclopropylmethylamines as selective serotonin reuptake inhibitors. J Med Chem. 2005 Sep 22;48(19):6023-34. [16162005 ]
- Tatsumi M, Groshan K, Blakely RD, Richelson E: Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997 Dec 11;340(2-3):249-58. [9537821 ]
- General Function:
- Phosphatidylinositol phospholipase c activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM1
- Uniprot ID:
- P11229
- Molecular Weight:
- 51420.375 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.072 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.3 uM | Not Available | BindingDB 22416 |
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Owens JM, Knight DL, Nemeroff CB: [Second generation SSRIS: human monoamine transporter binding profile of escitalopram and R-fluoxetine]. Encephale. 2002 Jul-Aug;28(4):350-5. [12232544 ]
- Stanton T, Bolden-Watson C, Cusack B, Richelson E: Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol. 1993 Jun 9;45(11):2352-4. [8100134 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Gene Name:
- HTR1A
- Uniprot ID:
- P08908
- Molecular Weight:
- 46106.335 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 22416 |
| Dissociation | 30 uM | Not Available | BindingDB 22416 |
References
- Langham JJ, Cleves AE, Spitzer R, Kirshner D, Jain AN: Physical binding pocket induction for affinity prediction. J Med Chem. 2009 Oct 8;52(19):6107-25. doi: 10.1021/jm901096y. [19754201 ]
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including ergot alkaloid derivatives, 1-2,5,-dimethoxy-4-iodophenyl-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Regulates neuronal activity via the activation of short transient receptor potential calcium channels in the brain, and thereby modulates the activation of pro-opiomelacortin neurons and the release of CRH that then regulates the release of corticosterone. Plays a role in the regulation of appetite and eating behavior, responses to anxiogenic stimuli and stress. Plays a role in insulin sensitivity and glucose homeostasis.
- Gene Name:
- HTR2C
- Uniprot ID:
- P28335
- Molecular Weight:
- 51820.705 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 22416 |
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM: RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997 Apr-May;36(4-5):621-9. [9225287 ]
- General Function:
- Thioesterase binding
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins. The rank order of potency for agonists of this receptor is oxymetazoline > clonidine > epinephrine > norepinephrine > phenylephrine > dopamine > p-synephrine > p-tyramine > serotonin = p-octopamine. For antagonists, the rank order is yohimbine > phentolamine = mianserine > chlorpromazine = spiperone = prazosin > propanolol > alprenolol = pindolol.
- Gene Name:
- ADRA2A
- Uniprot ID:
- P08913
- Molecular Weight:
- 48956.275 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 3.915 uM | Not Available | BindingDB 22416 |
| Inhibitory | >10 uM | Not Available | BindingDB 22416 |
References
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB: Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997 Dec;283(3):1305-22. [9400006 ]
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- General Function:
- G-protein coupled acetylcholine receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is adenylate cyclase inhibition. Signaling promotes phospholipase C activity, leading to the release of inositol trisphosphate (IP3); this then triggers calcium ion release into the cytosol.
- Gene Name:
- CHRM2
- Uniprot ID:
- P08172
- Molecular Weight:
- 51714.605 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.108 uM | Not Available | BindingDB 22416 |
| Inhibitory | 0.34 uM | Not Available | BindingDB 22416 |
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Stanton T, Bolden-Watson C, Cusack B, Richelson E: Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol. 1993 Jun 9;45(11):2352-4. [8100134 ]
- General Function:
- Receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM3
- Uniprot ID:
- P20309
- Molecular Weight:
- 66127.445 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.08 uM | Not Available | BindingDB 22416 |
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Stanton T, Bolden-Watson C, Cusack B, Richelson E: Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol. 1993 Jun 9;45(11):2352-4. [8100134 ]
- General Function:
- Guanyl-nucleotide exchange factor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is inhibition of adenylate cyclase.
- Gene Name:
- CHRM4
- Uniprot ID:
- P08173
- Molecular Weight:
- 53048.65 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.32 uM | Not Available | BindingDB 22416 |
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Stanton T, Bolden-Watson C, Cusack B, Richelson E: Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol. 1993 Jun 9;45(11):2352-4. [8100134 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various ergot alkaloid derivatives and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of dopamine and 5-hydroxytryptamine release, 5-hydroxytryptamine uptake and in the regulation of extracellular dopamine and 5-hydroxytryptamine levels, and thereby affects neural activity. May play a role in the perception of pain. Plays a role in the regulation of behavior, including impulsive behavior. Required for normal proliferation of embryonic cardiac myocytes and normal heart development. Protects cardiomyocytes against apoptosis. Plays a role in the adaptation of pulmonary arteries to chronic hypoxia. Plays a role in vasoconstriction. Required for normal osteoblast function and proliferation, and for maintaining normal bone density. Required for normal proliferation of the interstitial cells of Cajal in the intestine.
- Gene Name:
- HTR2B
- Uniprot ID:
- P41595
- Molecular Weight:
- 54297.41 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 22416 |
References
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM: RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997 Apr-May;36(4-5):621-9. [9225287 ]
- General Function:
- Steroid hydroxylase activity
- Specific Function:
- Responsible for the metabolism of many drugs and environmental chemicals that it oxidizes. It is involved in the metabolism of drugs such as antiarrhythmics, adrenoceptor antagonists, and tricyclic antidepressants.
- Gene Name:
- CYP2D6
- Uniprot ID:
- P10635
- Molecular Weight:
- 55768.94 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 4.85 uM | Not Available | BindingDB 22416 |
References
- Fontana E, Dansette PM, Poli SM: Cytochrome p450 enzymes mechanism based inhibitors: common sub-structures and reactivity. Curr Drug Metab. 2005 Oct;6(5):413-54. [16248836 ]
- General Function:
- Histamine receptor activity
- Specific Function:
- In peripheral tissues, the H1 subclass of histamine receptors mediates the contraction of smooth muscles, increase in capillary permeability due to contraction of terminal venules, and catecholamine release from adrenal medulla, as well as mediating neurotransmission in the central nervous system.
- Gene Name:
- HRH1
- Uniprot ID:
- P35367
- Molecular Weight:
- 55783.61 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 22416 |
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- General Function:
- Phosphatidylinositol phospholipase c activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM5
- Uniprot ID:
- P08912
- Molecular Weight:
- 60073.205 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Receptor for ATP that acts as a ligand-gated ion channel. This receptor is insensitive to the antagonists PPADS and suramin.
- Gene Name:
- P2RX4
- Uniprot ID:
- Q99571
- Molecular Weight:
- 43368.725 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 1.87 uM | Not Available | BindingDB 22416 |
References
- Hernandez-Olmos V, Abdelrahman A, El-Tayeb A, Freudendahl D, Weinhausen S, Muller CE: N-substituted phenoxazine and acridone derivatives: structure-activity relationships of potent P2X4 receptor antagonists. J Med Chem. 2012 Nov 26;55(22):9576-88. doi: 10.1021/jm300845v. Epub 2012 Nov 1. [23075067 ]
- General Function:
- Tachykinin receptor activity
- Specific Function:
- This is a receptor for the tachykinin neuropeptide substance P. It is probably associated with G proteins that activate a phosphatidylinositol-calcium second messenger system. The rank order of affinity of this receptor to tachykinins is: substance P > substance K > neuromedin-K.
- Gene Name:
- TACR1
- Uniprot ID:
- P25103
- Molecular Weight:
- 46250.5 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.9 uM | Not Available | BindingDB 22416 |
References
- Wu YJ, He H, Bertekap R, Westphal R, Lelas S, Newton A, Wallace T, Taber M, Davis C, Macor JE, Bronson J: Discovery of disubstituted piperidines and homopiperidines as potent dual NK1 receptor antagonists-serotonin reuptake transporter inhibitors for the treatment of depression. Bioorg Med Chem. 2013 Apr 15;21(8):2217-28. doi: 10.1016/j.bmc.2013.02.010. Epub 2013 Feb 19. [23477943 ]