| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:27:56 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2927 |
|---|
| Identification |
|---|
| Common Name | Cocaine |
|---|
| Class | Small Molecule |
|---|
| Description | An alkaloid ester extracted from the leaves of plants including coca. It is a local anesthetic and vasoconstrictor and is clinically used for that purpose, particularly in the eye, ear, nose, and throat. It also has powerful central nervous system effects similar to the amphetamines and is a drug of abuse. Cocaine, like amphetamines, acts by multiple mechanisms on brain catecholaminergic neurons; the mechanism of its reinforcing effects is thought to involve inhibition of dopamine uptake. [PubChem] |
|---|
| Compound Type | - Amine
- Anesthetic
- Anesthetic, Local
- Dopamine Uptake Inhibitor
- Drug
- Ester
- Ether
- Metabolite
- Organic Compound
- Synthetic Compound
- Vasoconstrictor Agent
|
|---|
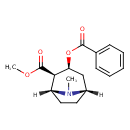
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (-)-Cocaine | | (−)-cocaine | | 2-Methyl-3beta-hydroxy-1alphah,5alphah-tropane-2beta-carboxylate benzoate (ester) | | Benzoylmethylecgonine | | beta-Cocain | | Cocain | | Cocaina | | Cocainum | | Kokain | | L-Cocain | | L-Cocaine | | Methyl benzoylecgonine | | Methyl [1R-(exo,exo)]-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | | Neurocaine | | [1R-(Exo,exo)]-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic acid, methyl ester |
|
|---|
| Chemical Formula | C17H21NO4 |
|---|
| Average Molecular Mass | 303.353 g/mol |
|---|
| Monoisotopic Mass | 303.147 g/mol |
|---|
| CAS Registry Number | 50-36-2 |
|---|
| IUPAC Name | methyl (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
|---|
| Traditional Name | cocaine |
|---|
| SMILES | [H][C@@]12CC[C@@]([H])(N1C)[C@@]([H])(C(=O)OC)[C@]([H])(C2)OC(=O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C17H21NO4/c1-18-12-8-9-13(18)15(17(20)21-2)14(10-12)22-16(19)11-6-4-3-5-7-11/h3-7,12-15H,8-10H2,1-2H3/t12-,13+,14-,15+/m0/s1 |
|---|
| InChI Key | InChIKey=ZPUCINDJVBIVPJ-LJISPDSOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoic acid esters. These are ester derivatives of benzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Benzoic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzoate ester
- Piperidinecarboxylic acid
- Tropane alkaloid
- Benzoyl
- Dicarboxylic acid or derivatives
- Piperidine
- N-alkylpyrrolidine
- Methyl ester
- Pyrrolidine
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary aliphatic amine
- Tertiary amine
- Carboxylic acid derivative
- Azacycle
- Organoheterocyclic compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Amine
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 98°C | | Boiling Point | Not Available | | Solubility | 1800 mg/L (at 22°C) | | LogP | 2.3 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-001i-9200000000-5262d40bbcdec08759ab | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0udi-0109000000-319d789d066a157a0966 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-001i-9200000000-5262d40bbcdec08759ab | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-0udi-0109000000-319d789d066a157a0966 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a5a-9620000000-fd84236e0a4ca8f55456 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0009000000-a2004e33ce1140fd0ed1 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0f89-0908000000-b5abe87da56f09f21f65 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-001i-0900000000-ee9c7f3b491476f49e98 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-001i-0900000000-2dab4b98f61ec4ba2467 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0fz9-0900000000-b3c61e257fbaf700b52b | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-001i-0900000000-3b1dc1a8c8390bfb6c3e | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0009000000-0479d3211208134ed140 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0ue9-0709000000-d19680d406708cbbc59b | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-001i-1900000000-5ac8f8a2719f70332b86 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-001i-3900000000-5ff2f6c28b5db03035be | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-053r-8900000000-e597f9f6f5014d501e62 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0a5c-9500000000-0fcf099229f148719078 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-0009000000-ee11934dd1158606bb84 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0ue9-0609000000-bd90cd1f98ce1845ba6b | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-001i-1900000000-9fc49ca16ab794b8c37d | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-001i-3900000000-cb0b593e7f379f7c6aef | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-053r-8900000000-2a88bad614aed29eac52 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0a59-9500000000-c9713abdcf52a45d6ed2 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-001i-0900000000-c18cf276038cf1946f20 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0859000000-d8747974615dabc86511 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ac0-0921000000-20e84a94ac21e65e27c3 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4900000000-1e0f166133422838c089 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0319000000-e9a39c150cc22fe3585e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0umi-4955000000-dea1abe1f68405b6dcd2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fi9-3900000000-c6cb0f24abf4c5d1446d | 2016-08-03 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Cocaine is absorbed from all sites of application, including mucous membranes and gastrointestinal mucosa. By oral or intra-nasal route, 60 to 80% of cocaine is absorbed. |
|---|
| Mechanism of Toxicity | Cocaine produces anesthesia by inhibiting excitation of nerve endings or by blocking conduction in peripheral nerves. This is achieved by reversibly binding to and inactivating sodium channels. Sodium influx through these channels is necessary for the depolarization of nerve cell membranes and subsequent propagation of impulses along the course of the nerve. Cocaine is the only local anesthetic with vasoconstrictive properties. This is a result of its blockade of norepinephrine reuptake in the autonomic nervous system. Cocaine binds differentially to the dopamine, serotonin, and norepinephrine transport proteins and directly prevents the re-uptake of dopamine, serotonin, and norepinephrine into pre-synaptic neurons. Its effect on dopamine levels is most responsible for the addictive property of cocaine. |
|---|
| Metabolism | Hepatic. Cocaine is metabolized to benzoylecgonine and ecgonine methyl ester, which are both excreted in the urine. In the presence of alcohol, a further active metabolite, cocaethylene is formed, and is more toxic then cocaine itself.
Half Life: 1 hour |
|---|
| Toxicity Values | Oral mouse LD50 = 96 mg/kg
LD50: 95.1 mg/kg (i.p, mouse) (8) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Cocaine (KOE-kane) is a local anesthetic. It is applied to certain areas of the body (for example, the nose, mouth, or throat) to cause loss of feeling. This allows some kinds of examinations or surgery to be done without causing pain. (7) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Continued use produces insomnia, hyperactivity, anxiousness, agitation and malnutrition. Overdoses can be lethal. |
|---|
| Symptoms | Intense agitation, convulsions, hypertension, rhythm disturbance, coronary insufficiency, hyperthermia, rhabdomyolysis, and renal impairment. |
|---|
| Treatment | The specific treatment of acute cocaine poisoning is the intravenous administration of a short-acting barbiturate or diazepam. Artificial respiration may be necessary. It is important to limit absorption of the drug. If entrance of the drug into circulation can be checked, and respiratory exchange maintained, the prognosis is favorable since cocaine is eliminated fairly rapidly. (9) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00907 |
|---|
| HMDB ID | HMDB15043 |
|---|
| PubChem Compound ID | 446220 |
|---|
| ChEMBL ID | CHEMBL120901 |
|---|
| ChemSpider ID | 10194104 |
|---|
| KEGG ID | C01416 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 27958 |
|---|
| BioCyc ID | CPD-9776 |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Cocaine |
|---|
| PDB ID | COC |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Cocaine |
|---|
| References |
|---|
| Synthesis Reference | Nobuyuki Shigetoh, Hiroshi Nakayama, Jinsei Miyazaki, Tadayasu Mitsumata, “Labelling colors for detecting cocaine or methamphetamine, method of preparing the same and detector for cocaine or methamphetamine.” U.S. Patent US5571727, issued October, 1981. |
|---|
| MSDS | Link |
|---|
| General References | - Siegel RK, Elsohly MA, Plowman T, Rury PM, Jones RT: Cocaine in herbal tea. JAMA. 1986 Jan 3;255(1):40. [3940302 ]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan J, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N: Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000 Aug 11;67(12):1507-15. [10983846 ]
- Dimitrijevic N, Dzitoyeva S, Manev H: An automated assay of the behavioral effects of cocaine injections in adult Drosophila. J Neurosci Methods. 2004 Aug 30;137(2):181-4. [15262059 ]
- Uz T, Akhisaroglu M, Ahmed R, Manev H: The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003 Dec;28(12):2117-23. [12865893 ]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ: Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9377-81. Epub 2005 Jun 20. [15967985 ]
- Busse GD, Riley AL: Effects of alcohol on cocaine lethality in rats: acute and chronic assessments. Neurotoxicol Teratol. 2003 May-Jun;25(3):361-4. [12757832 ]
- Drugs.com [Link]
- Erowid: Material Safety Data Sheets/ Psychoactive LD50s [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|