Melatonin (T3D2972)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:28:16 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:54 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2972 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Melatonin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Melatonin is a biogenic amine that is found in animals, plants and microbes. Aaron B. Lerner of Yale University is credited for naming the hormone and for defining its chemical structure in 1958. In mammals, melatonin is produced by the pineal gland. The pineal gland is small endocrine gland, about the size of a rice grain and shaped like a pine cone (hence the name), that is located in the center of the brain (rostro-dorsal to the superior colliculus) but outside the blood-brain barrier. The secretion of melatonin increases in darkness and decreases during exposure to light, thereby regulating the circadian rhythms of several biological functions, including the sleep-wake cycle. In particular, melatonin regulates the sleep-wake cycle by chemically causing drowsiness and lowering the body temperature. Melatonin is also implicated in the regulation of mood, learning and memory, immune activity, dreaming, fertility and reproduction. Melatonin is also an effective antioxidant. Most of the actions of melatonin are mediated through the binding and activation of melatonin receptors. Individuals with autism spectrum disorders (ASD) may have lower than normal levels of melatonin. A 2008 study found that unaffected parents of individuals with ASD also have lower melatonin levels, and that the deficits were associated with low activity of the ASMT gene, which encodes the last enzyme of melatonin synthesis. Reduced melatonin production has also been proposed as a likely factor in the significantly higher cancer rates in night workers. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
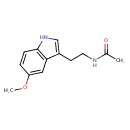
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C13H16N2O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 232.278 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 232.121 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 73-31-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | melatonin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | COC1=CC2=C(NC=C2CCN=C(C)O)C=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=DRLFMBDRBRZALE-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as 3-alkylindoles. 3-alkylindoles are compounds containing an indole moiety that carries an alkyl chain at the 3-position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Indoles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Indoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 3-alkylindoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Endogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | The absorption and bioavailability of melatonin varies widely. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Melatonin is a derivative of tryptophan. It binds to melatonin receptor type 1A, which then acts on adenylate cylcase and the inhibition of a cAMP signal transduction pathway. Melatonin not only inhibits adenylate cyclase, but it also activates phosphilpase C. This potentiates the release of arachidonate. By binding to melatonin receptors 1 and 2, the downstream signallling cascades have various effects in the body. The melatonin receptors are G protein-coupled receptors and are expressed in various tissues of the body. There are two subtypes of the receptor in humans, melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2). Melatonin and melatonin receptor agonists, on market or in clinical trials, all bind to and activate both receptor types.The binding of the agonists to the receptors has been investigated for over two decades or since 1986. It is somewhat known, but still not fully understood. When melatonin receptor agonists bind to and activate their receptors it causes numerous physiological processes. MT1 receptors are expressed in many regions of the central nervous system (CNS): suprachiasmatic nucleus of the hypothalamus (SNC), hippocampus, substantia nigra, cerebellum, central dopaminergic pathways, ventral tegmental area and nucleus accumbens. MT1 is also expressed in the retina, ovary, testis, mammary gland, coronary circulation and aorta, gallbladder, liver, kidney, skin and the immune system. MT2 receptors are expressed mainly in the CNS, also in the lung, cardiac, coronary and aortic tissue, myometrium and granulosa cells, immune cells, duodenum and adipocytes. The binding of melatonin to melatonin receptors activates a few signaling pathways. MT1 receptor activation inhibits the adenylyl cyclase and its inhibition causes a rippling effect of non activation; starting with decreasing formation of cyclic adenosine monophosphate (cAMP), and then progressing to less protein kinase A (PKA) activity, which in turn hinders the phosphorilation of cAMP responsive element-binding protein (CREB binding protein) into P-CREB. MT1 receptors also activate phospholipase C (PLC), affect ion channels and regulate ion flux inside the cell. The binding of melatonin to MT2 receptors inhibits adenylyl cyclase which decreases the formation of cAMP.[4] As well it hinders guanylyl cyclase and therefore the forming of cyclic guanosine monophosphate (cGMP). Binding to MT2 receptors probably affects PLC which increases protein kinase C (PKC) activity. Activation of the receptor can lead to ion flux inside the cell. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Hepatically metabolized to at least 14 identified metabolites (identified in mouse urine): 6-hydroxymelatonin glucuronide, 6-hydroxymelatonin sulfate, N-acetylserotonin glucuronide, N-acetylserotonin sulfate, 6-hydroxymelatonin, 2-oxomelatonin, 3-hydroxymelatonin, melatonin glucuronide, cyclic melatonin, cyclic N-acetylserotonin glucuronide, cyclic 6-hydroxymelatonin, 5-hydroxyindole-3-acetaldehyde, di-hydroxymelatonin and its glucuronide conjugate. 6-Hydroxymelatonin glucuronide is the major metabolite found in mouse urine (65-88% of total melatonin metabolites in urine). Half Life: 35 to 50 minutes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 3200 mg/kg (Oral, Rat) (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Used orally for jet lag, insomnia, shift-work disorder, circadian rhythm disorders in the blind (evidence for efficacy), and benzodiazepine and nicotine withdrawal. Evidence indicates that melatonin is likely effective for treating circadian rhythm sleep disorders in blind children and adults. It has received FDA orphan drug status as an oral medication for this use. A number of studies have shown that melatonin may be effective for treating sleep-wake cycle disturbances in children and adolescents with mental retardation, autism, and other central nervous system disorders. It appears to decrease the time to fall asleep in children with developmental disabilities, such as cerebral palsy, autism, and mental retardation. It may also improve secondary insomnia associated with various sleep-wake cycle disturbances. Other possible uses for which there is some evidence for include: benzodiazepine withdrawal, cluster headache, delayed sleep phase syndrome (DSPS), primary insomnia, jet lag, nicotine withdrawal, preoperative anxiety and sedation, prostate cancer, solid tumors (when combined with IL-2 therapy in certain cancers), sunburn prevention (topical use), tardive dyskinesia, thrombocytopenia associated with cancer, chemotherapy and other disorders. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Tolerance can develop, in which the person needs larger doses to achieve the desired effect; this can lead to overdose and death. Accidents or injury can also occur due to the side effects of loss of coordination, slowed reaction time, sleepiness and impaired judgment. Drugs in this category have a high potential for physical and psychological dependence. May cause a potentially dangerous rash that may develop into Stevens Johnson syndrome, an extremely rare but potentially fatal skin disease. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Loss of coordination, slowed reaction time, sleepiness and impaired judgment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB01065 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB01389 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 896 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 872 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C01598 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | 303800 , 303900 , 600950 , 604348 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 16796 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | N-ACETYL-5-METHOXY-TRYPTAMINE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Melatonin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | ML1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Melatonin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Robert A. S. Welch, Keith Betteridge, “Method of stimulating cashmere growth on cashmere-producing goats using melatonin.” U.S. Patent US4855313, issued August, 1986. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Melatonin receptor activity
- Specific Function:
- High affinity receptor for melatonin. Likely to mediates the reproductive and circadian actions of melatonin. The activity of this receptor is mediated by pertussis toxin sensitive G proteins that inhibit adenylate cyclase activity.
- Gene Name:
- MTNR1B
- Uniprot ID:
- P49286
- Molecular Weight:
- 40187.895 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.00012 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00018 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00019 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000195 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00021 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00023 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00025 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.0003 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00031 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00032 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00033 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000339 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00034 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00035 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00038 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00041 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000429 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00048 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.0005 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00053 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000617 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.0007 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000741 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00111 uM | Not Available | BindingDB 9019 |
| IC50 | 0.0003 uM | Not Available | BindingDB 9019 |
| IC50 | 0.00053 uM | Not Available | BindingDB 9019 |
References
- Radogna F, Paternoster L, De Nicola M, Cerella C, Ammendola S, Bedini A, Tarzia G, Aquilano K, Ciriolo M, Ghibelli L: Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol Appl Pharmacol. 2009 Aug 15;239(1):37-45. doi: 10.1016/j.taap.2009.05.012. Epub 2009 May 19. [19463840 ]
- Boutin JA, Audinot V, Ferry G, Delagrange P: Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005 Aug;26(8):412-9. [15992934 ]
- Hardeland R: Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009 Mar-Apr;35(2):183-92. doi: 10.1002/biof.23. [19449447 ]
- Srinivasan V, Singh J, Pandi-Perumal SR, Brown GM, Spence DW, Cardinali DP: Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010 Nov;27(11):796-813. doi: 10.1007/s12325-010-0065-y. Epub 2010 Sep 6. [20827520 ]
- Carocci A, Catalano A, Lovece A, Lentini G, Duranti A, Lucini V, Pannacci M, Scaglione F, Franchini C: Design, synthesis, and pharmacological effects of structurally simple ligands for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem. 2010 Sep 1;18(17):6496-511. doi: 10.1016/j.bmc.2010.06.100. Epub 2010 Jul 3. [20674373 ]
- Prendergast BJ: MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters (Phodopus sungorus). Endocrinology. 2010 Feb;151(2):714-21. doi: 10.1210/en.2009-0710. Epub 2009 Dec 4. [19966183 ]
- Mattson RJ, Catt JD, Keavy D, Sloan CP, Epperson J, Gao Q, Hodges DB, Iben L, Mahle CD, Ryan E, Yocca FD: Indanyl piperazines as melatonergic MT2 selective agents. Bioorg Med Chem Lett. 2003 Mar 24;13(6):1199-202. [12643943 ]
- Ettaoussi M, Peres B, Klupsch F, Delagrange P, Boutin JA, Renard P, Caignard DH, Chavatte P, Berthelot P, Lesieur D, Yous S: Design and synthesis of benzofuranic derivatives as new ligands at the melatonin-binding site MT3. Bioorg Med Chem. 2008 May 1;16(9):4954-62. doi: 10.1016/j.bmc.2008.03.036. Epub 2008 Mar 17. [18372181 ]
- Leclerc V, Ettaoussi M, Rami M, Farce A, Boutin JA, Delagrange P, Caignard DH, Renard P, Berthelot P, Yous S: Design and synthesis of naphthalenic derivatives as new ligands at the melatonin binding site MT3. Eur J Med Chem. 2011 May;46(5):1622-9. doi: 10.1016/j.ejmech.2011.02.010. Epub 2011 Feb 15. [21377769 ]
- Witt-Enderby PA, Chu GH, Gillen ML, Li PK: Development of a high-affinity ligand that binds irreversibly to Mel1b melatonin receptors. J Med Chem. 1997 Dec 19;40(26):4195-8. [9435890 ]
- Dubocovich ML, Masana MI, Iacob S, Sauri DM: Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol. 1997 Mar;355(3):365-75. [9089668 ]
- Beresford IJ, Browning C, Starkey SJ, Brown J, Foord SM, Coughlan J, North PC, Dubocovich ML, Hagan RM: GR196429: a nonindolic agonist at high-affinity melatonin receptors. J Pharmacol Exp Ther. 1998 Jun;285(3):1239-45. [9618428 ]
- Uchikawa O, Fukatsu K, Tokunoh R, Kawada M, Matsumoto K, Imai Y, Hinuma S, Kato K, Nishikawa H, Hirai K, Miyamoto M, Ohkawa S: Synthesis of a novel series of tricyclic indan derivatives as melatonin receptor agonists. J Med Chem. 2002 Sep 12;45(19):4222-39. [12213063 ]
- Koike T, Hoashi Y, Takai T, Nakayama M, Yukuhiro N, Ishikawa T, Hirai K, Uchikawa O: 1,6-Dihydro-2H-indeno[5,4-b]furan derivatives: design, synthesis, and pharmacological characterization of a novel class of highly potent MT(2)-selective agonists. J Med Chem. 2011 May 12;54(9):3436-44. doi: 10.1021/jm200221q. Epub 2011 Apr 7. [21473625 ]
- Koike T, Takai T, Hoashi Y, Nakayama M, Kosugi Y, Nakashima M, Yoshikubo S, Hirai K, Uchikawa O: Synthesis of a novel series of tricyclic dihydrofuran derivatives: discovery of 8,9-dihydrofuro[3,2-c]pyrazolo[1,5-a]pyridines as melatonin receptor (MT1/MT2) ligands. J Med Chem. 2011 Jun 23;54(12):4207-18. doi: 10.1021/jm200385u. Epub 2011 May 24. [21568291 ]
- Reppert SM, Weaver DR, Ebisawa T: Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994 Nov;13(5):1177-85. [7946354 ]
- Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM: Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists. Br J Pharmacol. 1999 Jul;127(5):1288-94. [10455277 ]
- Sun LQ, Chen J, Mattson RJ, Epperson JR, Deskus JA, Li WS, Takaki K, Hodges DB, Iben L, Mahle CD, Ortiz A, Molstad D, Ryan E, Yeleswaram K, Xu C, Luo G: Heterocyclic aminopyrrolidine derivatives as melatoninergic agents. Bioorg Med Chem Lett. 2003 Dec 15;13(24):4381-4. [14643330 ]
- Sun LQ, Chen J, Takaki K, Johnson G, Iben L, Mahle CD, Ryan E, Xu C: Design and synthesis of benzoxazole derivatives as novel melatoninergic ligands. Bioorg Med Chem Lett. 2004 Mar 8;14(5):1197-200. [14980664 ]
- Sun LQ, Chen J, Bruce M, Deskus JA, Epperson JR, Takaki K, Johnson G, Iben L, Mahle CD, Ryan E, Xu C: Synthesis and structure-activity relationship of novel benzoxazole derivatives as melatonin receptor agonists. Bioorg Med Chem Lett. 2004 Jul 16;14(14):3799-802. [15203165 ]
- Di Giacomo B, Bedini A, Spadoni G, Tarzia G, Fraschini F, Pannacci M, Lucini V: Synthesis and biological activity of new melatonin dimeric derivatives. Bioorg Med Chem. 2007 Jul 1;15(13):4643-50. Epub 2007 Mar 30. [17481904 ]
- Leclerc V, Yous S, Delagrange P, Boutin JA, Renard P, Lesieur D: Synthesis of nitroindole derivatives with high affinity and selectivity for melatoninergic binding sites MT(3). J Med Chem. 2002 Apr 25;45(9):1853-9. [11960497 ]
- Wallez V, Durieux-Poissonnier S, Chavatte P, Boutin JA, Audinot V, Nicolas JP, Bennejean C, Delagrange P, Renard P, Lesieur D: Synthesis and structure-affinity-activity relationships of novel benzofuran derivatives as MT(2) melatonin receptor selective ligands. J Med Chem. 2002 Jun 20;45(13):2788-800. [12061881 ]
- Epperson JR, Deskus JA, Gentile AJ, Iben LG, Ryan E, Sarbin NS: 4-Substituted anilides as selective melatonin MT2 receptor agonists. Bioorg Med Chem Lett. 2004 Feb 23;14(4):1023-6. [15013015 ]
- Sugden D, Pickering H, Teh MT, Garratt PJ: Melatonin receptor pharmacology: toward subtype specificity. Biol Cell. 1997 Nov;89(8):531-7. [9618903 ]
- Teh MT, Sugden D: Comparison of the structure-activity relationships of melatonin receptor agonists and antagonists: lengthening the N-acyl side-chain has differing effects on potency on Xenopus melanophores. Naunyn Schmiedebergs Arch Pharmacol. 1998 Nov;358(5):522-8. [9840420 ]
- Faust R, Garratt PJ, Jones R, Yeh LK, Tsotinis A, Panoussopoulou M, Calogeropoulou T, Teh MT, Sugden D: Mapping the melatonin receptor. 6. Melatonin agonists and antagonists derived from 6H-isoindolo[2,1-a]indoles, 5,6-dihydroindolo[2,1-a]isoquinolines, and 6,7-dihydro-5H-benzo[c]azepino[2,1-a]indoles. J Med Chem. 2000 Mar 23;43(6):1050-61. [10737738 ]
- Spadoni G, Bedini A, Orlando P, Lucarini S, Tarzia G, Mor M, Rivara S, Lucini V, Pannacci M, Scaglione F: Bivalent ligand approach on N-{2-[(3-methoxyphenyl)methylamino]ethyl}acetamide: synthesis, binding affinity and intrinsic activity for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem. 2011 Aug 15;19(16):4910-6. doi: 10.1016/j.bmc.2011.06.063. Epub 2011 Jun 28. [21775151 ]
- Jeanty M, Suzenet F, Delagrange P, Nosjean O, Boutin JA, Caignard DH, Guillaumet G: Design and synthesis of 1-(2-alkanamidoethyl)-6-methoxy-7-azaindole derivatives as potent melatonin agonists. Bioorg Med Chem Lett. 2011 Apr 15;21(8):2316-9. doi: 10.1016/j.bmcl.2011.02.097. Epub 2011 Feb 26. [21420861 ]
- El Kazzouli S, Griffon du Bellay A, Berteina-Raboin S, Delagrange P, Caignard DH, Guillaumet G: Design and synthesis of 2-phenylimidazo[1,2-a]pyridines as a novel class of melatonin receptor ligands. Eur J Med Chem. 2011 Sep;46(9):4252-7. doi: 10.1016/j.ejmech.2011.06.030. Epub 2011 Jul 1. [21764185 ]
- Tsotinis A, Vlachou M, Papahatjis DP, Calogeropoulou T, Nikas SP, Garratt PJ, Piccio V, Vonhoff S, Davidson K, Teh MT, Sugden D: Mapping the melatonin receptor. 7. Subtype selective ligands based on beta-substituted N-acyl-5-methoxytryptamines and beta-substituted N-acyl-5-methoxy-1-methyltryptamines. J Med Chem. 2006 Jun 15;49(12):3509-19. [16759094 ]
- Mesangeau C, Fraise M, Delagrange P, Caignard DH, Boutin JA, Berthelot P, Yous S: Preparation and pharmacological evaluation of a novel series of 2-(phenylthio)benzo[b]thiophenes as selective MT2 receptor ligands. Eur J Med Chem. 2011 May;46(5):1835-40. doi: 10.1016/j.ejmech.2011.02.044. Epub 2011 Feb 23. [21392858 ]
- Descamps-Francois C, Yous S, Chavatte P, Audinot V, Bonnaud A, Boutin JA, Delagrange P, Bennejean C, Renard P, Lesieur D: Design and synthesis of naphthalenic dimers as selective MT1 melatoninergic ligands. J Med Chem. 2003 Mar 27;46(7):1127-9. [12646022 ]
- Poissonnier-Durieux S, Ettaoussi M, Peres B, Boutin JA, Audinot V, Bennejean C, Delagrange P, Caignard DH, Renard P, Berthelot P, Lesieur D, Yous S: Synthesis of 3-phenylnaphthalenic derivatives as new selective MT(2) melatoninergic ligands. Bioorg Med Chem. 2008 Sep 15;16(18):8339-48. doi: 10.1016/j.bmc.2008.08.052. Epub 2008 Aug 27. [18778943 ]
- Hu Y, Ho MK, Chan KH, New DC, Wong YH: Synthesis of substituted N-[3-(3-methoxyphenyl)propyl] amides as highly potent MT(2)-selective melatonin ligands. Bioorg Med Chem Lett. 2010 Apr 15;20(8):2582-5. doi: 10.1016/j.bmcl.2010.02.084. Epub 2010 Feb 25. [20227878 ]
- Hu Y, Zhu J, Chan KH, Wong YH: Development of substituted N-[3-(3-methoxylphenyl)propyl] amides as MT(2)-selective melatonin agonists: improving metabolic stability. Bioorg Med Chem. 2013 Jan 15;21(2):547-52. doi: 10.1016/j.bmc.2012.10.060. Epub 2012 Nov 20. [23228808 ]
- Morellato L, Lefas-Le Gall M, Langlois M, Caignard DH, Renard P, Delagrange P, Mathe-Allainmat M: Synthesis of new N-(arylcyclopropyl)acetamides and N-(arylvinyl)acetamides as conformationally-restricted ligands for melatonin receptors. Bioorg Med Chem Lett. 2013 Jan 15;23(2):430-4. doi: 10.1016/j.bmcl.2012.11.069. Epub 2012 Nov 29. [23265885 ]
- Jellimann C, Mathe-Allainmat M, Andrieux J, Kloubert S, Boutin JA, Nicolas JP, Bennejean C, Delagrange P, Langlois M: Synthesis of phenalene and acenaphthene derivatives as new conformationally restricted ligands for melatonin receptors. J Med Chem. 2000 Nov 2;43(22):4051-62. [11063602 ]
- Sun LQ, Takaki K, Chen J, Iben L, Knipe JO, Pajor L, Mahle CD, Ryan E, Xu C: N-[2-[2-(4-Phenylbutyl)benzofuran-4-yl]cyclopropylmethyl]acetamide: an orally bioavailable melatonin receptor agonist. Bioorg Med Chem Lett. 2004 Oct 18;14(20):5157-60. [15380218 ]
- Sun LQ, Takaki K, Chen J, Bertenshaw S, Iben L, Mahle CD, Ryan E, Wu D, Gao Q, Xu C: (R)-2-(4-Phenylbutyl)dihydrobenzofuran derivatives as melatoninergic agents. Bioorg Med Chem Lett. 2005 Mar 1;15(5):1345-9. [15713384 ]
- Attia MI, Witt-Enderby PA, Julius J: Synthesis and pharmacological evaluation of pentacyclic 6a,7-dihydrodiindole and 2,3-dihydrodiindole derivatives as novel melatoninergic ligands. Bioorg Med Chem. 2008 Aug 15;16(16):7654-61. doi: 10.1016/j.bmc.2008.07.012. Epub 2008 Jul 10. [18657980 ]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF: Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8734-8. [7568007 ]
- Radogna F, Paternoster L, Albertini MC, Cerella C, Accorsi A, Bucchini A, Spadoni G, Diamantini G, Tarzia G, De Nicola M, D'Alessio M, Ghibelli L: Melatonin antagonizes apoptosis via receptor interaction in U937 monocytic cells. J Pineal Res. 2007 Sep;43(2):154-62. [17645693 ]
- General Function:
- Organic cyclic compound binding
- Specific Function:
- High affinity receptor for melatonin. Likely to mediates the reproductive and circadian actions of melatonin. The activity of this receptor is mediated by pertussis toxin sensitive G proteins that inhibit adenylate cyclase activity.
- Gene Name:
- MTNR1A
- Uniprot ID:
- P48039
- Molecular Weight:
- 39374.315 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.00008 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.0000823 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00012 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00014 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.0002 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00022 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00024 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00025 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000263 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000296 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.0003 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000331 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00037 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00039 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.0004 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.000525 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00053 uM | Not Available | BindingDB 9019 |
| Inhibitory | 0.00066 uM | Not Available | BindingDB 9019 |
| IC50 | 0.0002 uM | Not Available | BindingDB 9019 |
| IC50 | 0.0006 uM | Not Available | BindingDB 9019 |
| IC50 | 0.001 uM | Not Available | BindingDB 9019 |
References
- Schuster C, Williams LM, Morris A, Morgan PJ, Barrett P: The human MT1 melatonin receptor stimulates cAMP production in the human neuroblastoma cell line SH-SY5Y cells via a calcium-calmodulin signal transduction pathway. J Neuroendocrinol. 2005 Mar;17(3):170-8. [15796769 ]
- Tomas-Zapico C, Antonio Boga J, Caballero B, Vega-Naredo I, Sierra V, Alvarez-Garcia O, Tolivia D, Josefa Rodriguez-Colunga M, Coto-Montes A: Coexpression of MT1 and RORalpha1 melatonin receptors in the Syrian hamster Harderian gland. J Pineal Res. 2005 Aug;39(1):21-6. [15978053 ]
- Wu YH, Zhou JN, Balesar R, Unmehopa U, Bao A, Jockers R, Van Heerikhuize J, Swaab DF: Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J Comp Neurol. 2006 Dec 20;499(6):897-910. [17072839 ]
- Park YJ, Park JG, Hiyakawa N, Lee YD, Kim SJ, Takemura A: Diurnal and circadian regulation of a melatonin receptor, MT1, in the golden rabbitfish, Siganus guttatus. Gen Comp Endocrinol. 2007 Jan 15;150(2):253-62. Epub 2006 Oct 16. [17046760 ]
- Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ: Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006 Feb 15;68(6):425-9. Epub 2005 Oct 12. [16459197 ]
- Ettaoussi M, Peres B, Klupsch F, Delagrange P, Boutin JA, Renard P, Caignard DH, Chavatte P, Berthelot P, Lesieur D, Yous S: Design and synthesis of benzofuranic derivatives as new ligands at the melatonin-binding site MT3. Bioorg Med Chem. 2008 May 1;16(9):4954-62. doi: 10.1016/j.bmc.2008.03.036. Epub 2008 Mar 17. [18372181 ]
- Leclerc V, Ettaoussi M, Rami M, Farce A, Boutin JA, Delagrange P, Caignard DH, Renard P, Berthelot P, Yous S: Design and synthesis of naphthalenic derivatives as new ligands at the melatonin binding site MT3. Eur J Med Chem. 2011 May;46(5):1622-9. doi: 10.1016/j.ejmech.2011.02.010. Epub 2011 Feb 15. [21377769 ]
- Mattson RJ, Catt JD, Keavy D, Sloan CP, Epperson J, Gao Q, Hodges DB, Iben L, Mahle CD, Ryan E, Yocca FD: Indanyl piperazines as melatonergic MT2 selective agents. Bioorg Med Chem Lett. 2003 Mar 24;13(6):1199-202. [12643943 ]
- Marot C, Chavatte P, Morin-Allory L, Viaud MC, Guillaumet G, Renard P, Lesieur D, Michel A: Pharmacophoric search and 3D-QSAR comparative molecular field analysis studies on agonists of melatonin sheep receptors. J Med Chem. 1998 Nov 5;41(23):4453-65. [9804685 ]
- Witt-Enderby PA, Chu GH, Gillen ML, Li PK: Development of a high-affinity ligand that binds irreversibly to Mel1b melatonin receptors. J Med Chem. 1997 Dec 19;40(26):4195-8. [9435890 ]
- Uchikawa O, Fukatsu K, Tokunoh R, Kawada M, Matsumoto K, Imai Y, Hinuma S, Kato K, Nishikawa H, Hirai K, Miyamoto M, Ohkawa S: Synthesis of a novel series of tricyclic indan derivatives as melatonin receptor agonists. J Med Chem. 2002 Sep 12;45(19):4222-39. [12213063 ]
- Leclerc V, Yous S, Delagrange P, Boutin JA, Renard P, Lesieur D: Synthesis of nitroindole derivatives with high affinity and selectivity for melatoninergic binding sites MT(3). J Med Chem. 2002 Apr 25;45(9):1853-9. [11960497 ]
- Wallez V, Durieux-Poissonnier S, Chavatte P, Boutin JA, Audinot V, Nicolas JP, Bennejean C, Delagrange P, Renard P, Lesieur D: Synthesis and structure-affinity-activity relationships of novel benzofuran derivatives as MT(2) melatonin receptor selective ligands. J Med Chem. 2002 Jun 20;45(13):2788-800. [12061881 ]
- Descamps-Francois C, Yous S, Chavatte P, Audinot V, Bonnaud A, Boutin JA, Delagrange P, Bennejean C, Renard P, Lesieur D: Design and synthesis of naphthalenic dimers as selective MT1 melatoninergic ligands. J Med Chem. 2003 Mar 27;46(7):1127-9. [12646022 ]
- Poissonnier-Durieux S, Ettaoussi M, Peres B, Boutin JA, Audinot V, Bennejean C, Delagrange P, Caignard DH, Renard P, Berthelot P, Lesieur D, Yous S: Synthesis of 3-phenylnaphthalenic derivatives as new selective MT(2) melatoninergic ligands. Bioorg Med Chem. 2008 Sep 15;16(18):8339-48. doi: 10.1016/j.bmc.2008.08.052. Epub 2008 Aug 27. [18778943 ]
- Jellimann C, Mathe-Allainmat M, Andrieux J, Kloubert S, Boutin JA, Nicolas JP, Bennejean C, Delagrange P, Langlois M: Synthesis of phenalene and acenaphthene derivatives as new conformationally restricted ligands for melatonin receptors. J Med Chem. 2000 Nov 2;43(22):4051-62. [11063602 ]
- Di Giacomo B, Bedini A, Spadoni G, Tarzia G, Fraschini F, Pannacci M, Lucini V: Synthesis and biological activity of new melatonin dimeric derivatives. Bioorg Med Chem. 2007 Jul 1;15(13):4643-50. Epub 2007 Mar 30. [17481904 ]
- Morellato L, Lefas-Le Gall M, Langlois M, Caignard DH, Renard P, Delagrange P, Mathe-Allainmat M: Synthesis of new N-(arylcyclopropyl)acetamides and N-(arylvinyl)acetamides as conformationally-restricted ligands for melatonin receptors. Bioorg Med Chem Lett. 2013 Jan 15;23(2):430-4. doi: 10.1016/j.bmcl.2012.11.069. Epub 2012 Nov 29. [23265885 ]
- Mesangeau C, Fraise M, Delagrange P, Caignard DH, Boutin JA, Berthelot P, Yous S: Preparation and pharmacological evaluation of a novel series of 2-(phenylthio)benzo[b]thiophenes as selective MT2 receptor ligands. Eur J Med Chem. 2011 May;46(5):1835-40. doi: 10.1016/j.ejmech.2011.02.044. Epub 2011 Feb 23. [21392858 ]
- Koike T, Hoashi Y, Takai T, Nakayama M, Yukuhiro N, Ishikawa T, Hirai K, Uchikawa O: 1,6-Dihydro-2H-indeno[5,4-b]furan derivatives: design, synthesis, and pharmacological characterization of a novel class of highly potent MT(2)-selective agonists. J Med Chem. 2011 May 12;54(9):3436-44. doi: 10.1021/jm200221q. Epub 2011 Apr 7. [21473625 ]
- Koike T, Takai T, Hoashi Y, Nakayama M, Kosugi Y, Nakashima M, Yoshikubo S, Hirai K, Uchikawa O: Synthesis of a novel series of tricyclic dihydrofuran derivatives: discovery of 8,9-dihydrofuro[3,2-c]pyrazolo[1,5-a]pyridines as melatonin receptor (MT1/MT2) ligands. J Med Chem. 2011 Jun 23;54(12):4207-18. doi: 10.1021/jm200385u. Epub 2011 May 24. [21568291 ]
- Jeanty M, Suzenet F, Delagrange P, Nosjean O, Boutin JA, Caignard DH, Guillaumet G: Design and synthesis of 1-(2-alkanamidoethyl)-6-methoxy-7-azaindole derivatives as potent melatonin agonists. Bioorg Med Chem Lett. 2011 Apr 15;21(8):2316-9. doi: 10.1016/j.bmcl.2011.02.097. Epub 2011 Feb 26. [21420861 ]
- El Kazzouli S, Griffon du Bellay A, Berteina-Raboin S, Delagrange P, Caignard DH, Guillaumet G: Design and synthesis of 2-phenylimidazo[1,2-a]pyridines as a novel class of melatonin receptor ligands. Eur J Med Chem. 2011 Sep;46(9):4252-7. doi: 10.1016/j.ejmech.2011.06.030. Epub 2011 Jul 1. [21764185 ]
- Spadoni G, Bedini A, Orlando P, Lucarini S, Tarzia G, Mor M, Rivara S, Lucini V, Pannacci M, Scaglione F: Bivalent ligand approach on N-{2-[(3-methoxyphenyl)methylamino]ethyl}acetamide: synthesis, binding affinity and intrinsic activity for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem. 2011 Aug 15;19(16):4910-6. doi: 10.1016/j.bmc.2011.06.063. Epub 2011 Jun 28. [21775151 ]
- Hu Y, Ho MK, Chan KH, New DC, Wong YH: Synthesis of substituted N-[3-(3-methoxyphenyl)propyl] amides as highly potent MT(2)-selective melatonin ligands. Bioorg Med Chem Lett. 2010 Apr 15;20(8):2582-5. doi: 10.1016/j.bmcl.2010.02.084. Epub 2010 Feb 25. [20227878 ]
- Hu Y, Zhu J, Chan KH, Wong YH: Development of substituted N-[3-(3-methoxylphenyl)propyl] amides as MT(2)-selective melatonin agonists: improving metabolic stability. Bioorg Med Chem. 2013 Jan 15;21(2):547-52. doi: 10.1016/j.bmc.2012.10.060. Epub 2012 Nov 20. [23228808 ]
- Sun LQ, Takaki K, Chen J, Iben L, Knipe JO, Pajor L, Mahle CD, Ryan E, Xu C: N-[2-[2-(4-Phenylbutyl)benzofuran-4-yl]cyclopropylmethyl]acetamide: an orally bioavailable melatonin receptor agonist. Bioorg Med Chem Lett. 2004 Oct 18;14(20):5157-60. [15380218 ]
- Sun LQ, Takaki K, Chen J, Bertenshaw S, Iben L, Mahle CD, Ryan E, Wu D, Gao Q, Xu C: (R)-2-(4-Phenylbutyl)dihydrobenzofuran derivatives as melatoninergic agents. Bioorg Med Chem Lett. 2005 Mar 1;15(5):1345-9. [15713384 ]
- Carocci A, Catalano A, Lovece A, Lentini G, Duranti A, Lucini V, Pannacci M, Scaglione F, Franchini C: Design, synthesis, and pharmacological effects of structurally simple ligands for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem. 2010 Sep 1;18(17):6496-511. doi: 10.1016/j.bmc.2010.06.100. Epub 2010 Jul 3. [20674373 ]
- Dolusic E, Larrieu P, Moineaux L, Stroobant V, Pilotte L, Colau D, Pochet L, Van den Eynde B, Masereel B, Wouters J, Frederick R: Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J Med Chem. 2011 Aug 11;54(15):5320-34. doi: 10.1021/jm2006782. Epub 2011 Jul 18. [21726069 ]
- Tsotinis A, Vlachou M, Papahatjis DP, Calogeropoulou T, Nikas SP, Garratt PJ, Piccio V, Vonhoff S, Davidson K, Teh MT, Sugden D: Mapping the melatonin receptor. 7. Subtype selective ligands based on beta-substituted N-acyl-5-methoxytryptamines and beta-substituted N-acyl-5-methoxy-1-methyltryptamines. J Med Chem. 2006 Jun 15;49(12):3509-19. [16759094 ]
- Sun LQ, Chen J, Mattson RJ, Epperson JR, Deskus JA, Li WS, Takaki K, Hodges DB, Iben L, Mahle CD, Ortiz A, Molstad D, Ryan E, Yeleswaram K, Xu C, Luo G: Heterocyclic aminopyrrolidine derivatives as melatoninergic agents. Bioorg Med Chem Lett. 2003 Dec 15;13(24):4381-4. [14643330 ]
- Sun LQ, Chen J, Takaki K, Johnson G, Iben L, Mahle CD, Ryan E, Xu C: Design and synthesis of benzoxazole derivatives as novel melatoninergic ligands. Bioorg Med Chem Lett. 2004 Mar 8;14(5):1197-200. [14980664 ]
- Sun LQ, Chen J, Bruce M, Deskus JA, Epperson JR, Takaki K, Johnson G, Iben L, Mahle CD, Ryan E, Xu C: Synthesis and structure-activity relationship of novel benzoxazole derivatives as melatonin receptor agonists. Bioorg Med Chem Lett. 2004 Jul 16;14(14):3799-802. [15203165 ]
- Attia MI, Witt-Enderby PA, Julius J: Synthesis and pharmacological evaluation of pentacyclic 6a,7-dihydrodiindole and 2,3-dihydrodiindole derivatives as novel melatoninergic ligands. Bioorg Med Chem. 2008 Aug 15;16(16):7654-61. doi: 10.1016/j.bmc.2008.07.012. Epub 2008 Jul 10. [18657980 ]
- Epperson JR, Deskus JA, Gentile AJ, Iben LG, Ryan E, Sarbin NS: 4-Substituted anilides as selective melatonin MT2 receptor agonists. Bioorg Med Chem Lett. 2004 Feb 23;14(4):1023-6. [15013015 ]
- Faust R, Garratt PJ, Jones R, Yeh LK, Tsotinis A, Panoussopoulou M, Calogeropoulou T, Teh MT, Sugden D: Mapping the melatonin receptor. 6. Melatonin agonists and antagonists derived from 6H-isoindolo[2,1-a]indoles, 5,6-dihydroindolo[2,1-a]isoquinolines, and 6,7-dihydro-5H-benzo[c]azepino[2,1-a]indoles. J Med Chem. 2000 Mar 23;43(6):1050-61. [10737738 ]
- Radogna F, Paternoster L, Albertini MC, Cerella C, Accorsi A, Bucchini A, Spadoni G, Diamantini G, Tarzia G, De Nicola M, D'Alessio M, Ghibelli L: Melatonin antagonizes apoptosis via receptor interaction in U937 monocytic cells. J Pineal Res. 2007 Sep;43(2):154-62. [17645693 ]
- General Function:
- Ubiquitin protein ligase binding
- Specific Function:
- Suppresses apoptosis in a variety of cell systems including factor-dependent lymphohematopoietic and neural cells. Regulates cell death by controlling the mitochondrial membrane permeability. Appears to function in a feedback loop system with caspases. Inhibits caspase activity either by preventing the release of cytochrome c from the mitochondria and/or by binding to the apoptosis-activating factor (APAF-1). May attenuate inflammation by impairing NLRP1-inflammasome activation, hence CASP1 activation and IL1B release (PubMed:17418785).
- Gene Name:
- BCL2
- Uniprot ID:
- P10415
- Molecular Weight:
- 26265.66 Da
References
- Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I, Kim J, Kim EH: Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci Lett. 2005 May 20-27;380(1-2):26-31. Epub 2005 Jan 25. [15854745 ]
- Trubiani O, Recchioni R, Moroni F, Pizzicannella J, Caputi S, Di Primio R: Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathway activation. J Pineal Res. 2005 Nov;39(4):425-31. [16207299 ]
- Baydas G, Reiter RJ, Akbulut M, Tuzcu M, Tamer S: Melatonin inhibits neural apoptosis induced by homocysteine in hippocampus of rats via inhibition of cytochrome c translocation and caspase-3 activation and by regulating pro- and anti-apoptotic protein levels. Neuroscience. 2005;135(3):879-86. [16213988 ]
- Yang QH, Xu JN, Xu RK, Pang SF: Inhibitory effects of melatonin on the growth of pituitary prolactin-secreting tumor in rats. J Pineal Res. 2006 Apr;40(3):230-5. [16499559 ]
- Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P: Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: involvement of nuclear factor kappa B, Bax and Bcl-2. J Pineal Res. 2006 Sep;41(2):116-23. [16879316 ]
- General Function:
- Phospholipase a2 activator activity
- Specific Function:
- Involved in the activation cascade of caspases responsible for apoptosis execution. At the onset of apoptosis it proteolytically cleaves poly(ADP-ribose) polymerase (PARP) at a '216-Asp-|-Gly-217' bond. Cleaves and activates sterol regulatory element binding proteins (SREBPs) between the basic helix-loop-helix leucine zipper domain and the membrane attachment domain. Cleaves and activates caspase-6, -7 and -9. Involved in the cleavage of huntingtin. Triggers cell adhesion in sympathetic neurons through RET cleavage.
- Gene Name:
- CASP3
- Uniprot ID:
- P42574
- Molecular Weight:
- 31607.58 Da
References
- Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I, Kim J, Kim EH: Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci Lett. 2005 May 20-27;380(1-2):26-31. Epub 2005 Jan 25. [15854745 ]
- Baydas G, Reiter RJ, Akbulut M, Tuzcu M, Tamer S: Melatonin inhibits neural apoptosis induced by homocysteine in hippocampus of rats via inhibition of cytochrome c translocation and caspase-3 activation and by regulating pro- and anti-apoptotic protein levels. Neuroscience. 2005;135(3):879-86. [16213988 ]
- Juknat AA, Mendez Mdel V, Quaglino A, Fameli CI, Mena M, Kotler ML: Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J Pineal Res. 2005 Mar;38(2):84-92. [15683462 ]
- Wenzel U, Nickel A, Daniel H: Melatonin potentiates flavone-induced apoptosis in human colon cancer cells by increasing the level of glycolytic end products. Int J Cancer. 2005 Aug 20;116(2):236-42. [15800915 ]
- Feng Z, Zhang JT: Long-term melatonin or 17beta-estradiol supplementation alleviates oxidative stress in ovariectomized adult rats. Free Radic Biol Med. 2005 Jul 15;39(2):195-204. Epub 2005 Mar 25. [15964511 ]
- General Function:
- Metal ion binding
- Specific Function:
- Electron carrier protein. The oxidized form of the cytochrome c heme group can accept an electron from the heme group of the cytochrome c1 subunit of cytochrome reductase. Cytochrome c then transfers this electron to the cytochrome oxidase complex, the final protein carrier in the mitochondrial electron-transport chain.Plays a role in apoptosis. Suppression of the anti-apoptotic members or activation of the pro-apoptotic members of the Bcl-2 family leads to altered mitochondrial membrane permeability resulting in release of cytochrome c into the cytosol. Binding of cytochrome c to Apaf-1 triggers the activation of caspase-9, which then accelerates apoptosis by activating other caspases.
- Gene Name:
- CYCS
- Uniprot ID:
- P99999
- Molecular Weight:
- 11748.69 Da
References
- Baydas G, Reiter RJ, Akbulut M, Tuzcu M, Tamer S: Melatonin inhibits neural apoptosis induced by homocysteine in hippocampus of rats via inhibition of cytochrome c translocation and caspase-3 activation and by regulating pro- and anti-apoptotic protein levels. Neuroscience. 2005;135(3):879-86. [16213988 ]
- Yang QH, Xu JN, Xu RK, Pang SF: Inhibitory effects of melatonin on the growth of pituitary prolactin-secreting tumor in rats. J Pineal Res. 2006 Apr;40(3):230-5. [16499559 ]
- Lin AM, Fang SF, Chao PL, Yang CH: Melatonin attenuates arsenite-induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res. 2007 Sep;43(2):163-71. [17645694 ]
- Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A: A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry. 2005 Jul 5;44(26):9300-7. [15981996 ]
- Han YX, Zhang SH, Wang XM, Wu JB: Inhibition of mitochondria responsible for the anti-apoptotic effects of melatonin during ischemia-reperfusion. J Zhejiang Univ Sci B. 2006 Feb;7(2):142-7. [16421971 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear hormone receptor. The steroid hormones and their receptors are involved in the regulation of eukaryotic gene expression and affect cellular proliferation and differentiation in target tissues. Ligand-dependent nuclear transactivation involves either direct homodimer binding to a palindromic estrogen response element (ERE) sequence or association with other DNA-binding transcription factors, such as AP-1/c-Jun, c-Fos, ATF-2, Sp1 and Sp3, to mediate ERE-independent signaling. Ligand binding induces a conformational change allowing subsequent or combinatorial association with multiprotein coactivator complexes through LXXLL motifs of their respective components. Mutual transrepression occurs between the estrogen receptor (ER) and NF-kappa-B in a cell-type specific manner. Decreases NF-kappa-B DNA-binding activity and inhibits NF-kappa-B-mediated transcription from the IL6 promoter and displace RELA/p65 and associated coregulators from the promoter. Recruited to the NF-kappa-B response element of the CCL2 and IL8 promoters and can displace CREBBP. Present with NF-kappa-B components RELA/p65 and NFKB1/p50 on ERE sequences. Can also act synergistically with NF-kappa-B to activate transcription involving respective recruitment adjacent response elements; the function involves CREBBP. Can activate the transcriptional activity of TFF1. Also mediates membrane-initiated estrogen signaling involving various kinase cascades. Isoform 3 is involved in activation of NOS3 and endothelial nitric oxide production. Isoforms lacking one or several functional domains are thought to modulate transcriptional activity by competitive ligand or DNA binding and/or heterodimerization with the full length receptor. Essential for MTA1-mediated transcriptional regulation of BRCA1 and BCAS3. Isoform 3 can bind to ERE and inhibit isoform 1.
- Gene Name:
- ESR1
- Uniprot ID:
- P03372
- Molecular Weight:
- 66215.45 Da
References
- Cos S, Martinez-Campa C, Mediavilla MD, Sanchez-Barcelo EJ: Melatonin modulates aromatase activity in MCF-7 human breast cancer cells. J Pineal Res. 2005 Mar;38(2):136-42. [15683469 ]
- Stevens RG: Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005 Mar;16(2):254-8. [15703542 ]
- Ishido M: Transient inhibition of synergistically insulin-like growth factor-1- and bisphenol A-induced poliferation of estrogen receptor alpha (ERalpha)-positive human breast cancer MCF-7 cells by melatonin. Environ Sci. 2004;11(3):163-70. [15750583 ]
- Cini G, Neri B, Pacini A, Cesati V, Sassoli C, Quattrone S, D'Apolito M, Fazio A, Scapagnini G, Provenzani A, Quattrone A: Antiproliferative activity of melatonin by transcriptional inhibition of cyclin D1 expression: a molecular basis for melatonin-induced oncostatic effects. J Pineal Res. 2005 Aug;39(1):12-20. [15978052 ]
- Martinez-Campa C, Alonso-Gonzalez C, Mediavilla MD, Cos S, Gonzalez A, Ramos S, Sanchez-Barcelo EJ: Melatonin inhibits both ER alpha activation and breast cancer cell proliferation induced by a metalloestrogen, cadmium. J Pineal Res. 2006 May;40(4):291-6. [16635015 ]
- General Function:
- Nadph dehydrogenase (quinone) activity
- Specific Function:
- The enzyme apparently serves as a quinone reductase in connection with conjugation reactions of hydroquinones involved in detoxification pathways as well as in biosynthetic processes such as the vitamin K-dependent gamma-carboxylation of glutamate residues in prothrombin synthesis.
- Gene Name:
- NQO2
- Uniprot ID:
- P16083
- Molecular Weight:
- 25918.4 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.06457 uM | Not Available | BindingDB 9019 |
| IC50 | 6.6 uM | Not Available | BindingDB 9019 |
| IC50 | 11.3 uM | Not Available | BindingDB 9019 |
References
- Radogna F, Paternoster L, De Nicola M, Cerella C, Ammendola S, Bedini A, Tarzia G, Aquilano K, Ciriolo M, Ghibelli L: Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol Appl Pharmacol. 2009 Aug 15;239(1):37-45. doi: 10.1016/j.taap.2009.05.012. Epub 2009 May 19. [19463840 ]
- Du H, Wang J, Zhang X, Hu Z: A novel quantitative structure-activity relationship method to predict the affinities of MT3 melatonin binding site. Eur J Med Chem. 2008 Dec;43(12):2861-9. doi: 10.1016/j.ejmech.2008.02.012. Epub 2008 Feb 29. [18400335 ]
- Volkova MS, Jensen KC, Lozinskaya NA, Sosonyuk SE, Proskurnina MV, Mesecar AD, Zefirov NS: Synthesis of novel capital EM, Cyrilliccapital TE, Cyrillic3 receptor ligands via an unusual Knoevenagel condensation. Bioorg Med Chem Lett. 2012 Dec 15;22(24):7578-81. doi: 10.1016/j.bmcl.2012.10.005. Epub 2012 Oct 17. [23131339 ]
- General Function:
- Virus receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including mescaline, psilocybin, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates phospholipase C and a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and promotes the release of Ca(2+) ions from intracellular stores. Affects neural activity, perception, cognition and mood. Plays a role in the regulation of behavior, including responses to anxiogenic situations and psychoactive substances. Plays a role in intestinal smooth muscle contraction, and may play a role in arterial vasoconstriction.(Microbial infection) Acts as a receptor for human JC polyomavirus/JCPyV.
- Gene Name:
- HTR2A
- Uniprot ID:
- P28223
- Molecular Weight:
- 52602.58 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 9019 |
References
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL: Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther. 1996 Feb;276(2):720-7. [8632342 ]
- Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D: The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003 Sep;306(3):954-64. Epub 2003 May 15. [12750432 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various ergot alkaloid derivatives and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of dopamine and 5-hydroxytryptamine release, 5-hydroxytryptamine uptake and in the regulation of extracellular dopamine and 5-hydroxytryptamine levels, and thereby affects neural activity. May play a role in the perception of pain. Plays a role in the regulation of behavior, including impulsive behavior. Required for normal proliferation of embryonic cardiac myocytes and normal heart development. Protects cardiomyocytes against apoptosis. Plays a role in the adaptation of pulmonary arteries to chronic hypoxia. Plays a role in vasoconstriction. Required for normal osteoblast function and proliferation, and for maintaining normal bone density. Required for normal proliferation of the interstitial cells of Cajal in the intestine.
- Gene Name:
- HTR2B
- Uniprot ID:
- P41595
- Molecular Weight:
- 54297.41 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 5.7544 uM | Not Available | BindingDB 9019 |
| Inhibitory | >10 uM | Not Available | BindingDB 9019 |
References
- Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D: The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003 Sep;306(3):954-64. Epub 2003 May 15. [12750432 ]
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL: Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther. 1996 Feb;276(2):720-7. [8632342 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including ergot alkaloid derivatives, 1-2,5,-dimethoxy-4-iodophenyl-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Regulates neuronal activity via the activation of short transient receptor potential calcium channels in the brain, and thereby modulates the activation of pro-opiomelacortin neurons and the release of CRH that then regulates the release of corticosterone. Plays a role in the regulation of appetite and eating behavior, responses to anxiogenic stimuli and stress. Plays a role in insulin sensitivity and glucose homeostasis.
- Gene Name:
- HTR2C
- Uniprot ID:
- P28335
- Molecular Weight:
- 51820.705 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 9019 |
References
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL: Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther. 1996 Feb;276(2):720-7. [8632342 ]
- Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D: The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003 Sep;306(3):954-64. Epub 2003 May 15. [12750432 ]
- General Function:
- Not Available
- Specific Function:
- Not Available
- Gene Name:
- SNCA
- Uniprot ID:
- P37840
- Molecular Weight:
- 14460.155 Da
References
- Ishido M: Melatonin inhibits maneb-induced aggregation of alpha-synuclein in rat pheochromocytoma cells. J Pineal Res. 2007 Mar;42(2):125-30. [17286743 ]
- Lin AM, Fang SF, Chao PL, Yang CH: Melatonin attenuates arsenite-induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res. 2007 Sep;43(2):163-71. [17645694 ]
- General Function:
- Titin binding
- Specific Function:
- Calmodulin mediates the control of a large number of enzymes, ion channels, aquaporins and other proteins by Ca(2+). Among the enzymes to be stimulated by the calmodulin-Ca(2+) complex are a number of protein kinases and phosphatases. Together with CCP110 and centrin, is involved in a genetic pathway that regulates the centrosome cycle and progression through cytokinesis.
- Gene Name:
- CALM1
- Uniprot ID:
- P0DP23
- Molecular Weight:
- 16837.47 Da
References
- del Rio B, Garcia Pedrero JM, Martinez-Campa C, Zuazua P, Lazo PS, Ramos S: Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004 Sep 10;279(37):38294-302. Epub 2004 Jun 30. [15229223 ]
- Radogna F, Paternoster L, De Nicola M, Cerella C, Ammendola S, Bedini A, Tarzia G, Aquilano K, Ciriolo M, Ghibelli L: Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol Appl Pharmacol. 2009 Aug 15;239(1):37-45. doi: 10.1016/j.taap.2009.05.012. Epub 2009 May 19. [19463840 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Calcium-binding chaperone that promotes folding, oligomeric assembly and quality control in the endoplasmic reticulum (ER) via the calreticulin/calnexin cycle. This lectin interacts transiently with almost all of the monoglucosylated glycoproteins that are synthesized in the ER. Interacts with the DNA-binding domain of NR3C1 and mediates its nuclear export. Involved in maternal gene expression regulation. May participate in oocyte maturation via the regulation of calcium homeostasis (By similarity).
- Gene Name:
- CALR
- Uniprot ID:
- P27797
- Molecular Weight:
- 48141.2 Da
References
- Hardeland R: Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009 Mar-Apr;35(2):183-92. doi: 10.1002/biof.23. [19449447 ]
- Macias M, Escames G, Leon J, Coto A, Sbihi Y, Osuna A, Acuna-Castroviejo D: Calreticulin-melatonin. An unexpected relationship. Eur J Biochem. 2003 Mar;270(5):832-40. [12603316 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Isoform 1 catalyzes the transfer of a methyl group onto N-acetylserotonin, producing melatonin (N-acetyl-5-methoxytryptamine). Isoform 2 and isoform 3 lack enzyme activity.
- Gene Name:
- ASMT
- Uniprot ID:
- P46597
- Molecular Weight:
- 38452.51 Da
References
- Minneman KP, Wurtman RJ: The pharmacology of the pineal gland. Annu Rev Pharmacol Toxicol. 1976;16:33-51. [180879 ]
- General Function:
- Peroxidase activity
- Specific Function:
- Mediates tyrosine nitration of secondary granule proteins in mature resting eosinophils. Shows significant inhibitory activity towards Mycobacterium tuberculosis H37Rv by inducing bacterial fragmentation and lysis.
- Gene Name:
- EPX
- Uniprot ID:
- P11678
- Molecular Weight:
- 81039.5 Da
References
- Lu T, Galijasevic S, Abdulhamid I, Abu-Soud HM: Analysis of the mechanism by which melatonin inhibits human eosinophil peroxidase. Br J Pharmacol. 2008 Jul;154(6):1308-17. doi: 10.1038/bjp.2008.173. Epub 2008 Jun 2. [18516076 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Binds heavy metals. Contains three zinc and three copper atoms per polypeptide chain and only a negligible amount of cadmium. Inhibits survival and neurite formation of cortical neurons in vitro.
- Gene Name:
- MT3
- Uniprot ID:
- P25713
- Molecular Weight:
- 6926.855 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.0646 uM | Not Available | BindingDB 9019 |
References
- Leclerc V, Ettaoussi M, Rami M, Farce A, Boutin JA, Delagrange P, Caignard DH, Renard P, Berthelot P, Yous S: Design and synthesis of naphthalenic derivatives as new ligands at the melatonin binding site MT3. Eur J Med Chem. 2011 May;46(5):1622-9. doi: 10.1016/j.ejmech.2011.02.010. Epub 2011 Feb 15. [21377769 ]
- General Function:
- Peroxidase activity
- Specific Function:
- Part of the host defense system of polymorphonuclear leukocytes. It is responsible for microbicidal activity against a wide range of organisms. In the stimulated PMN, MPO catalyzes the production of hypohalous acids, primarily hypochlorous acid in physiologic situations, and other toxic intermediates that greatly enhance PMN microbicidal activity.
- Gene Name:
- MPO
- Uniprot ID:
- P05164
- Molecular Weight:
- 83867.71 Da
References
- Galijasevic S, Abdulhamid I, Abu-Soud HM: Melatonin is a potent inhibitor for myeloperoxidase. Biochemistry. 2008 Feb 26;47(8):2668-77. doi: 10.1021/bi702016q. Epub 2008 Feb 1. [18237195 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear receptor that binds DNA as a monomer to ROR response elements (RORE) containing a single core motif half-site 5'-AGGTCA-3' preceded by a short A-T-rich sequence. Considered to have intrinsic transcriptional activity, have some natural ligands such as all-trans retinoic acid (ATRA) and other retinoids which act as inverse agonists repressing the transcriptional activity. Required for normal postnatal development of rod and cone photoreceptor cells. Modulates rod photoreceptors differentiation at least by inducing the transcription factor NRL-mediated pathway. In cone photoreceptor cells, regulates transcription of OPN1SW. Involved in the regulation of the period length and stability of the circadian rhythm. May control cytoarchitectural patterning of neocortical neurons during development. May act in a dose-dependent manner to regulate barrel formation upon innervation of layer IV neurons by thalamocortical axons. May play a role in the suppression of osteoblastic differentiation through the inhibition of RUNX2 transcriptional activity (By similarity).Isoform 1 is critical for hindlimb motor control and for the differentiation of amacrine and horizontal cells in the retina. Regulates the expression of PTF1A synergistically with FOXN4 (By similarity).
- Gene Name:
- RORB
- Uniprot ID:
- Q92753
- Molecular Weight:
- 53219.385 Da
References
- Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C: Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J Biol Chem. 1994 Nov 18;269(46):28531-4. [7961794 ]