| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-08-05 17:27:44 UTC |
|---|
| Update Date | 2014-12-24 20:26:10 UTC |
|---|
| Accession Number | T3D3571 |
|---|
| Identification |
|---|
| Common Name | Nitric acid |
|---|
| Class | Small Molecule |
|---|
| Description | Nitric acid (HNO3), also known as aqua fortis and spirit of niter, is a highly corrosive mineral acid. The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Most commercially available nitric acid has a concentration of 68%. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Nitric acid is the primary reagent used for nitration - the addition of a nitro group, typically to an organic molecule. The main industrial use of nitric acid is for the production of fertilizers. Nitric acid is neutralized with ammonia to give ammonium nitrate. The other main applications are for the production of explosives, nylon precursors, and specialty organic compounds. |
|---|
| Compound Type | - Household Toxin
- Industrial/Workplace Toxin
- Inorganic Compound
- Nitrate
- Nitrite
- Non-Metal
- Synthetic Compound
|
|---|
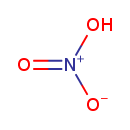
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Acide azotique | | Acide nitrique | | Acido nitrico | | Acidum nitricum | | Acidum Nitricum-Injeel Forte Liq (D6-D200) | | Aqua fortis | | Azotic acid | | Azotowy kwas | | Dynamite acid | | Engraver's acid | | Engravers acid | | Fuming nitric acid | | HNO3 | | HONO2 | | Hydrogen nitrate | | hydrogen trioxonitrate(1-) | | Hydroxidodioxidonitrogen | | Kyselina dusicne | | Nital | | Nitraline | | Nitrate | | Nitric acid (red fuming) | | Nitric acid anhydrous | | Nitric acid fuming | | Nitric acid red fuming | | Nitric acid standard solution | | Nitricum acidum | | Nitrous fumes | | Nitryl hydroxide | | Red fuming nitric acid | | RFNA | | Salpetersaeure | | Salpetersaure | | Salpeterzuuroplossingen | | Trioxonitric acid | | [NO2(OH)] |
|
|---|
| Chemical Formula | HNO3 |
|---|
| Average Molecular Mass | 63.013 g/mol |
|---|
| Monoisotopic Mass | 62.996 g/mol |
|---|
| CAS Registry Number | 7697-37-2 |
|---|
| IUPAC Name | nitric acid |
|---|
| Traditional Name | nitric acid |
|---|
| SMILES | O[N+]([O-])=O |
|---|
| InChI Identifier | InChI=1S/HNO3/c2-1(3)4/h(H,2,3,4) |
|---|
| InChI Key | InChIKey=GRYLNZFGIOXLOG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as non-metal nitrates. These are inorganic non-metallic compounds containing a nitrate as its largest oxoanion. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Non-metal oxoanionic compounds |
|---|
| Sub Class | Non-metal nitrates |
|---|
| Direct Parent | Non-metal nitrates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Non-metal nitrate
- Inorganic oxide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -42°C | | Boiling Point | 83°C | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9000000000-75a0abe959565212ac4f | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9000000000-8e1d753ca100f3f6d92d | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-9000000000-f295c80752f82f542bfa | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9000000000-59f156be4bb354eb31f7 | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-dbfc7b33c21beeeeb7e8 | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dj-9000000000-a596b228780b7e6ae195 | 2015-09-15 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (6) ; inhalation (6) |
|---|
| Mechanism of Toxicity | Nitric acid is a corrosive acid and a powerful oxidizing agent. The major hazard posed by it is chemical burns as it carries out acid hydrolysis with proteins (amide) and fats (ester) which consequently decomposes living tissue (e.g. skin and flesh). Concentrated nitric acid stains human skin yellow due to its reaction with the keratin. These yellow stains turn orange when neutralized. Systemic effects are unlikely, however, and the substance is not considered a carcinogen or mutagen. |
|---|
| Metabolism | Intake of some amount of nitrates and nitrites is a normal part of the nitrogen cycle in humans. In vivo conversion of nitrates to nitrites can occur in the gastrointestional tract under the right conditions, significantly enhancing nitrates' toxic potency. The major metabolic pathway for nitrate is conversion to nitrite, and then to ammonia. Nitrites, nitrates, and their metabolites are excreted in the urine. (6) |
|---|
| Toxicity Values | LD50: 138 ppm over 30 minutes (Inhalation, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Ingested nitrate or nitrite under conditions that result in endogenous nitrosation is probably carcinogenic to humans (Group 2A). (4) |
|---|
| Uses/Sources | The main industrial use of nitric acid is for the production of fertilizers. Nitric acid is neutralized with ammonia to give ammonium nitrate. The other main applications are for the production of explosives, nylon precursors, and specialty organic compounds. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Skin contact with nitric acid can cause redness, pain, and severe skin burns. Nitric acid may cause severe burns to the eye and permanent eye damage. Severe and rapid corrosive burns of the mouth, gullet and gastrointestinal tract will result if nitric acid is swallowed. Symptoms include burning, choking, nausea, vomiting and severe pain. |
|---|
| Symptoms | Skin contact can cause redness, pain, and severe skin burns. Nitric acid may cause severe burns to the eye and permanent eye damage. Severe and rapid corrosive burns of the mouth, gullet and gastrointestinal tract will result if nitric acid is swallowed. Symptoms include burning, choking, nausea, vomiting and severe pain.
|
|---|
| Treatment | The mainstay of treatment of any acid burn is copious irrigation with large amounts of tap water. To be most effective, treatment should be started immediately after exposure, preferably before arrival in the emergency department. Remove any contaminated clothing. Do not attempt to neutralize the burn with weak reciprocal chemicals (i.e. alkali for acid burns), because the heat generated from the chemical reaction may cause severe thermal injury. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 944 |
|---|
| ChEMBL ID | CHEMBL1352 |
|---|
| ChemSpider ID | 919 |
|---|
| KEGG ID | C00244 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 48107 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Nitric acid |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 6154 |
|---|
| Wikipedia Link | Nitric_acid |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3571.pdf |
|---|
| General References | - Keszler A, Piknova B, Schechter AN, Hogg N: The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008 Apr 11;283(15):9615-22. doi: 10.1074/jbc.M705630200. Epub 2008 Jan 17. [18203719 ]
- Park CH, Carboni E, Wood PL, Gee KW: Characterization of peripheral benzodiazepine type sites in a cultured murine BV-2 microglial cell line. Glia. 1996 Jan;16(1):65-70. [8787774 ]
- Environment Canada (1981). Tech Info for Problem Spills: Nitric acid.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1997). Toxicological profile for hydrazine. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2007). Case Studies in Environmental Medicine. Nitrate/Nitrite Toxicity. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Methemoglobinemia. Last Updated 22 July 2009. [Link]
- Wikipedia. Nitric acid. Last Updated August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|