| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2013-04-25 07:56:55 UTC |
|---|
| Update Date | 2014-12-24 20:26:34 UTC |
|---|
| Accession Number | T3D3936 |
|---|
| Identification |
|---|
| Common Name | Triasulfuron |
|---|
| Class | Small Molecule |
|---|
| Description | Triasulfuron is a selective sulfonylurea herbicide, which is absorbed by the leaves and the roots and is distributed through the plant to the meristems. It inhibits the biosynthesis of essential branched-chain amino acids valine and isoleucine, which prevents cell division and the plant growth is stopped. It is used as a herbicide in the cultivation of cereals (wheat, barley and triticale ) and can be applied before or after the emergence of the weeds. |
|---|
| Compound Type | - Amide
- Amine
- Ether
- Herbicide
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
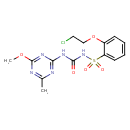
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1-[2-(2-Chloroethoxy)phenylsulfonyl]-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea | | 2-(2-Chloroethoxy)-N-{[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl}benzenesulfonamide | | Logran |
|
|---|
| Chemical Formula | C14H16ClN5O5S |
|---|
| Average Molecular Mass | 401.825 g/mol |
|---|
| Monoisotopic Mass | 401.056 g/mol |
|---|
| CAS Registry Number | 82097-50-5 |
|---|
| IUPAC Name | 1-[2-(2-chloroethoxy)benzenesulfonyl]-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea |
|---|
| Traditional Name | triasulfuron |
|---|
| SMILES | COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C2=CC=CC=C2OCCCl)=N1 |
|---|
| InChI Identifier | InChI=1S/C14H16ClN5O5S/c1-9-16-12(19-14(17-9)24-2)18-13(21)20-26(22,23)11-6-4-3-5-10(11)25-8-7-15/h3-6H,7-8H2,1-2H3,(H2,16,17,18,19,20,21) |
|---|
| InChI Key | InChIKey=XOPFESVZMSQIKC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzenesulfonamides. These are organic compounds containing a sulfonamide group that is S-linked to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Benzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenesulfonamide
- Benzenesulfonyl group
- Phenoxy compound
- 2-methoxy-1,3,5-triazine
- Alkoxy-s-triazine
- Phenol ether
- Alkyl aryl ether
- Triazine
- 1,3,5-triazine
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Sulfonyl
- Heteroaromatic compound
- Ether
- Organoheterocyclic compound
- Azacycle
- Organic oxide
- Alkyl halide
- Alkyl chloride
- Organic nitrogen compound
- Organopnictogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001l-1691200000-e55b0f9c5fc1ef0f0e3d | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0930000000-c974274aaf25a2675539 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-8900000000-1cb0305f895800c47ea0 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f79-1539500000-cf152b3002160c43d4cd | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056a-5793000000-bbde2e1153511e0256d8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9100000000-259633ea5d65a770d590 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 73282 |
|---|
| ChEMBL ID | CHEMBL1896787 |
|---|
| ChemSpider ID | 66025 |
|---|
| KEGG ID | C10961 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3936.pdf |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|