| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 04:49:18 UTC |
|---|
| Update Date | 2014-12-24 20:26:35 UTC |
|---|
| Accession Number | T3D4020 |
|---|
| Identification |
|---|
| Common Name | Colchicine |
|---|
| Class | Small Molecule |
|---|
| Description | Colchicine is only found in individuals that have used or taken this drug. It is a major alkaloid from Colchicum autumnale L. and found also in other Colchicum species. Its primary therapeutic use is in the treatment of gout, but it has been used also in the therapy of familial Mediterranean fever (periodic disease). The precise mechanism of action has not been completely established. In patients with gout, colchicine apparently interrupts the cycle of monosodium urate crystal deposition in joint tissues and the resultant inflammatory response that initiates and sustains an acute attack. Colchicine decreases leukocyte chemotaxis and phagocytosis and inhibits the formation and release of a chemotactic glycoprotein that is produced during phagocytosis of urate crystals. Colchicine also inhibits urate crystal deposition, which is enhanced by a low pH in the tissues, probably by inhibiting oxidation of glucose and subsequent lactic acid production in leukocytes. Colchicine has no analgesic or antihyperuricemic activity. Colchicine inhibits microtubule assembly in various cells, including leukocytes, probably by binding to and interfering with polymerization of the microtubule subunit tubulin. Although some studies have found that this action probably does not contribute significantly to colchicine's antigout action, a recent in vitro study has shown that it may be at least partially involved. |
|---|
| Compound Type | - Drug
- Ester
- Ether
- Gout Suppressant
- Metabolite
- Natural Compound
- Organic Compound
- Plant Toxin
- Tubulin Modulator
|
|---|
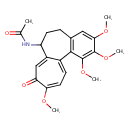
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Colchicin | | Colchicina | | Colchicinum | | Colcrys |
|
|---|
| Chemical Formula | C22H25NO6 |
|---|
| Average Molecular Mass | 399.437 g/mol |
|---|
| Monoisotopic Mass | 399.168 g/mol |
|---|
| CAS Registry Number | 64-86-8 |
|---|
| IUPAC Name | N-{3,4,5,14-tetramethoxy-13-oxotricyclo[9.5.0.0²,⁷]hexadeca-1(16),2(7),3,5,11,14-hexaen-10-yl}acetamide |
|---|
| Traditional Name | colchicine |
|---|
| SMILES | COC1=C(OC)C(OC)=C2C(CCC(N=C(C)O)C3=CC(=O)C(OC)=CC=C23)=C1 |
|---|
| InChI Identifier | InChI=1/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24) |
|---|
| InChI Key | InChIKey=IAKHMKGGTNLKSZ-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tropones. Tropones are compounds containing a tropone ring, which is a cycloheptatrienone ring bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbon derivatives |
|---|
| Class | Tropones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tropones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anisole
- Tropone
- Alkyl aryl ether
- Benzenoid
- Cyclic ketone
- Carboximidic acid
- Carboximidic acid derivative
- Ether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Organic oxide
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 156°C | | Boiling Point | Not Available | | Solubility | 4.5E+004 mg/L (at 25°C) | | LogP | 1.3 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053u-1009000000-7a8d292bb6a5673c9d7c | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0000900000-c73c5a28a750cece607a | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0049400000-2a76bd81d41e1bc5925c | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0gba-0091000000-fb24bc910ffe7bd74811 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-03gi-0096000000-1604b0f123208b25b949 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0zfr-0029400000-dc08a845d6fb327eec56 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-d360bbc1ca27b2e657d4 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000900000-44e9ac8566ca5c684603 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-9d973f7afeacb8db799d | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0gc1-0192000000-dcb2736f710df3f17b01 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0049500000-27383218cdc648739307 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0gba-0091000000-fb24bc910ffe7bd74811 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfr-0009700000-00c1abf086f6716cac6b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0009100000-4f6b3c625a6887981670 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05dl-0049000000-75fafbc5e28d11766c0c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-cc76299babee13af8502 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0537-1009000000-247faa52698339d3e544 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pi3-9077000000-eb1350fc19fa611668aa | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0001900000-958a463c4e2f7c3dd588 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udl-0009500000-8181c90153beb9e9a4f5 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052g-5079000000-81182c306cc4f86cf2c3 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-b40b49dac61f8e9864fa | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-7009000000-666c5fffe6918e3020f7 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9007000000-7e1ec907f14345d7e992 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Colchicine is rapidly absorbed after oral administration, probably from the jejunum and ileum. However, the rate and extent of absorption are variable, depending on the tablet dissolution rate; variability in gastric emptying, intestinal motility, and pH at the absorption site; and the extent to which colchicine is bound to microtubules in gastrointestinal mucosal cells. |
|---|
| Mechanism of Toxicity | Colchicine inhibits microtubule assembly in various cells, including leukocytes, probably by binding to and interfering with polymerization of the microtubule subunit tubulin. Although some studies have found that this action probably does not contribute significantly to colchicine's antigout action, a recent in vitro study has shown that it may be at least partially involved. |
|---|
| Metabolism | Probably hepatic. Although colchicine metabolites have not been identified in humans, metabolism by mammalian hepatic microsomes has been demonstrated in vitro.
Route of Elimination: In healthy volunteers (n=12) 40 - 65% of 1 mg orally administered colchicine was recovered unchanged in urine.
Enterohepatic recirculation and biliary excretion are also postulated to play a role in colchicine elimination.
Half Life: Elimination half-life is approximately 1 hour in healthy subjects, although a study with an extended sampling time reported mean terminal elimination half-life values of approximately 9 to 10.5 hours. Other studies have reported half-life values of approximately 2 hours in patients with alcoholic cirrhosis and approximately 2.5 hours in patients with familial Mediterranean fever. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For treatment and relief of pain in attacks of acute gouty arthritis. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01394 |
|---|
| HMDB ID | HMDB15466 |
|---|
| PubChem Compound ID | 2833 |
|---|
| ChEMBL ID | CHEMBL107 |
|---|
| ChemSpider ID | 2731 |
|---|
| KEGG ID | C07592 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 23359 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Colchicine |
|---|
| References |
|---|
| Synthesis Reference | Christian Wehrey, “Colchicine derivatives, process for preparing them, products obtained therefrom and use thereof.” U.S. Patent US20040138182, issued July 15, 2004. |
|---|
| MSDS | Link |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|