| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 05:47:31 UTC |
|---|
| Update Date | 2014-12-24 20:26:40 UTC |
|---|
| Accession Number | T3D4156 |
|---|
| Identification |
|---|
| Common Name | L-Cysteine |
|---|
| Class | Small Molecule |
|---|
| Description | Cysteine is a uremic toxin. Uremic toxins can be subdivided into three major groups based upon their chemical and physical characteristics: 1) small, water-soluble, non-protein-bound compounds, such as urea; 2) small, lipid-soluble and/or protein-bound compounds, such as the phenols and 3) larger so-called middle-molecules, such as beta2-microglobulin. Chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease.
Cysteine is a naturally occurring, sulfur-containing amino acid that is found in most proteins, although only in small quantities. Cysteine is unique amongst the twenty natural amino acids as it contains a thiol group. Thiol groups can undergo oxidation/reduction (redox) reactions; when cysteine is oxidized it can form cystine, which is two cysteine residues joined by a disulfide bond. This reaction is reversible: as reduction of this disulphide bond regenerates two cysteine molecules. The disulphide bonds of cystine are crucial to defining the structures of many proteins. Cysteine is often involved in electron-transfer reactions, and help the enzyme catalyze its reaction. Cysteine is also part of the antioxidant glutathione. N-acetyl-L-cysteine (NAC) is a form of cysteine where an acetyl group is attached to cysteine's nitrogen atom and is sold as a dietary supplement. Cysteine is named after cystine, which comes from the Greek word kustis meaning bladder - cystine was first isolated from kidney stones. As cysteine contains a sulphydryl group, it can undergo redox reactions. Oxidation of cysteine can produce a disulfide bond with another thiol, or further oxidation can produce sulphfinic or sulfonic acids. The cysteine thiol group is also a nucleophile and can undergo addition and substitution reactions. Thiol groups become much more reactive when they are ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell. The thiol group also has a high affinity for heavy metals and proteins containing cysteine will bind metals such as mercury, lead and cadmium tightly. Due to this ability to undergo redox reactions, cysteine has antioxidant properties. Cysteine is an important source of sulfur in human metabolism, and although it is classified as a non-essential amino acid, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine may at some point be recognized as an essential or conditionally essential amino acid. Cysteine is important in energy metabolism. As cystine, it is a structural component of many tissues and hormones. Cysteine has clinical uses ranging from baldness to psoriasis to preventing smoker's hack. In some cases, oral cysteine therapy has proved excellent for treatment of asthmatics, enabling them to stop theophylline and other medications. Cysteine also enhances the effect of topically applied silver, tin and zinc salts in preventing dental cavities. In the future, cysteine may play a role in the treatment of cobalt toxicity, diabetes, psychosis, cancer and seizures. |
|---|
| Compound Type | - Amine
- Dietary Supplement
- Drug
- Food Toxin
- Household Toxin
- Metabolite
- Natural Compound
- Nutraceutical
- Nutritional Support
- Organic Compound
- Supplement
- Uremic Toxin
|
|---|
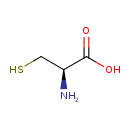
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (+)-2-Amino-3-mercaptopropionic acid | | (2R)-2-amino-3-mercaptopropanoate | | (2R)-2-amino-3-mercaptopropanoic acid | | (2R)-2-amino-3-sulfanylpropanoate | | (2R)-2-amino-3-sulfanylpropanoic acid | | (R)-(+)-cysteine | | (R)-2-amino-3-mercapto-Propanoate | | (R)-2-amino-3-mercapto-Propanoic acid | | (R)-2-Amino-3-mercaptopropanoate | | (R)-2-Amino-3-mercaptopropanoic acid | | (R)-cysteine | | 2-Amino-3-mercaptopropanoate | | 2-Amino-3-mercaptopropanoic acid | | 2-Amino-3-mercaptopropionate | | 2-Amino-3-mercaptopropionic acid | | 3-Mercapto-L-Alanine | | Acetylcysteine | | alpha-Amino-beta-thiolpropionic acid | | B-Mercaptoalanine | | beta-Mercaptoalanine | | Carbocysteine | | Cisteina | | Cisteinum | | Cys | | Cystein | | Cysteine | | Cysteinum | | Free cysteine | | Half-cystine | | L Cysteine | | L-(+)-Cysteine | | L-2-Amino-3-mercaptopropanoate | | L-2-Amino-3-mercaptopropanoic acid | | L-2-Amino-3-mercaptopropionic acid | | L-Cys | | L-Cystein | | Polycysteine | | Thioserine |
|
|---|
| Chemical Formula | C3H7NO2S |
|---|
| Average Molecular Mass | 121.158 g/mol |
|---|
| Monoisotopic Mass | 121.020 g/mol |
|---|
| CAS Registry Number | 52-90-4 |
|---|
| IUPAC Name | (2R)-2-amino-3-sulfanylpropanoic acid |
|---|
| Traditional Name | L-cysteine |
|---|
| SMILES | [H][C@](N)(CS)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C3H7NO2S/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1 |
|---|
| InChI Key | InChIKey=XUJNEKJLAYXESH-REOHCLBHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cysteine and derivatives. Cysteine and derivatives are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Cysteine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cysteine or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Amino acid
- Alkylthiol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
- Mitochondria
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Adrenal Cortex
- Epidermis
- Fibroblasts
- Intestine
- Kidney
- Liver
- Muscle
- Myelin
- Neuron
- Placenta
- Platelet
- Prostate
- Skeletal Muscle
- Spleen
- Testes
- Thyroid Gland
|
|---|
| Pathways | |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 240 dec°C | | Boiling Point | Not Available | | Solubility | 2.77E+005 mg/L (at 25°C) | | LogP | -2.49 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00kb-0950000000-df7e91c95b610ff21c79 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00kb-0940000000-aefe34765fb447090a23 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00kb-0970000000-10a155c40ea499023052 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-00kb-0940000000-037a3a34651c3154b3b3 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00di-9850000000-118c43e33861a6baa8d2 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014j-0690100000-0aeb88fd507505e6b718 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kb-0950000000-df7e91c95b610ff21c79 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kb-0940000000-aefe34765fb447090a23 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kb-0970000000-10a155c40ea499023052 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00kb-0940000000-037a3a34651c3154b3b3 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-QQ (Non-derivatized) | splash10-0uk9-5619100000-8cb2558373966174ced3 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9850000000-118c43e33861a6baa8d2 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0gi0-0960000000-03b2097de6f9637d29cb | 2017-09-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-9100000000-4553906a941a5e87ec97 | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004i-9200000000-cfaf705cd0452d428454 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00b9-9600000000-374c5872d68662832769 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4i-9000000000-ddbd3df6b8dbb280ffba | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0a4i-9000000000-320a2c77443b80ebf733 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0089-0900000000-9dcd3d757c5cd11eb18e | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-3900000000-212e081fe83ad70de0ad | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-9000000000-2eb01f41c225f614db24 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-001i-0900000000-a19834eb7cb9f211fdf2 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-00di-0900000000-2458f2587761e779ed93 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-9000000000-77e590f0ed26f69b31c0 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0udi-3900000000-7b1857997392b006b95f | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-3900000000-bc268c27ed5706a4bbd2 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - CE-ESI-TOF (CE-system connected to 6210 Time-of-Flight MS, Agilent) , Positive | splash10-00di-0900000000-4573390bccc238e3c91b | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-3900000000-212e081fe83ad70de0ad | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0udi-3900000000-7b1857997392b006b95f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 14V, negative | splash10-001i-9000000000-5dc69d3b59bd15290aef | 2020-07-21 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-9100000000-ed3c9c47ed98679090ab | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-00di-2900000000-8bb219d17ccdf7ac0a5d | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0a4i-9000000000-e623d5a27b5075c82b4d | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-056r-9100000000-4e26cc13af7c878115f7 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-9600000000-4352a7b437c34c04d9f0 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9200000000-7b4cd034c717561c61dd | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-8f351cbca6ffed356a9b | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-6900000000-51ba0df80cc33423420b | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-9400000000-4aa2b707c391d5838d29 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-95c7672c7836c0fc51c9 | 2016-09-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 125 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | Not Available | 2016-10-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | Not Available | 2021-10-10 | View Spectrum | | 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | Not Available | 2012-12-04 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Endogenous, Ingestion, Dermal (contact) |
|---|
| Mechanism of Toxicity | Uremic toxins such as cysteine are actively transported into the kidneys via organic ion transporters (especially OAT3). Increased levels of uremic toxins can stimulate the production of reactive oxygen species. This seems to be mediated by the direct binding or inhibition by uremic toxins of the enzyme NADPH oxidase (especially NOX4 which is abundant in the kidneys and heart) (2). Reactive oxygen species can induce several different DNA methyltransferases (DNMTs) which are involved in the silencing of a protein known as KLOTHO. KLOTHO has been identified as having important roles in anti-aging, mineral metabolism, and vitamin D metabolism. A number of studies have indicated that KLOTHO mRNA and protein levels are reduced during acute or chronic kidney diseases in response to high local levels of reactive oxygen species (3).
Although classified as a non-essential amino acid cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available. Due to the ability of thiols to undergo redox reactions, cysteine has antioxidant properties. Cysteine's antioxidant properties are typically expressed in the tripeptide glutathione, which occurs in humans as well as other organisms. The systemic availability of oral glutathione (GSH) is negligible; so it must be biosynthesized from its constituent amino acids, cysteine, glycine, and glutamic acid. Glutamic acid and glycine are readily available in the diets of most industrialized countries, but the availability of cysteine can be the limiting substrate. Cysteine is also an important source of sulfide in human metabolism. The sulfide in iron-sulfur clusters and in nitrogenase is extracted from cysteine, which is converted to alanine in the process. In a 1994 report released by five top cigarette companies, cysteine is one of the 599 additives to cigarettes. Its use or purpose, however, is unknown, like most cigarette additives. Its inclusion in cigarettes could offer two benefits: Acting as an expectorant, since smoking increases mucus production in the lungs; and increasing the beneficial antioxidant glutathione (which is diminished in smokers). |
|---|
| Metabolism | Uremic toxins tend to accumulate in the blood either through dietary excess or through poor filtration by the kidneys. Most uremic toxins are metabolic waste products and are normally excreted in the urine or feces. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the prevention of liver damage and kidney damage associated with overdoses of acetaminophen |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Chronic exposure to uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease. |
|---|
| Symptoms | As a uremic toxin, this compound can cause uremic syndrome. Uremic syndrome may affect any part of the body and can cause nausea, vomiting, loss of appetite, and weight loss. It can also cause changes in mental status, such as confusion, reduced awareness, agitation, psychosis, seizures, and coma. Abnormal bleeding, such as bleeding spontaneously or profusely from a very minor injury can also occur. Heart problems, such as an irregular heartbeat, inflammation in the sac that surrounds the heart (pericarditis), and increased pressure on the heart can be seen in patients with uremic syndrome. Shortness of breath from fluid buildup in the space between the lungs and the chest wall (pleural effusion) can also be present. |
|---|
| Treatment | Kidney dialysis is usually needed to relieve the symptoms of uremic syndrome until normal kidney function can be restored. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00151 |
|---|
| HMDB ID | HMDB00574 |
|---|
| PubChem Compound ID | 5862 |
|---|
| ChEMBL ID | CHEMBL863 |
|---|
| ChemSpider ID | 5653 |
|---|
| KEGG ID | C00097 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 17561 |
|---|
| BioCyc ID | CYS |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | CYS |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | L-Cysteine |
|---|
| References |
|---|
| Synthesis Reference | Alfred Maierhofer, Hans Wagner, “Process for the production of high purity S-carboxymethyl-L-cysteine.” U.S. Patent US4129593, issued May, 1965. |
|---|
| MSDS | Link |
|---|
| General References | - Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012 Jul;23(7):1258-70. doi: 10.1681/ASN.2011121175. Epub 2012 May 24. [22626821 ]
- Schulz AM, Terne C, Jankowski V, Cohen G, Schaefer M, Boehringer F, Tepel M, Kunkel D, Zidek W, Jankowski J: Modulation of NADPH oxidase activity by known uraemic retention solutes. Eur J Clin Invest. 2014 Aug;44(8):802-11. doi: 10.1111/eci.12297. [25041433 ]
- Young GH, Wu VC: KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 2012 Apr;81(7):611-2. doi: 10.1038/ki.2011.461. [22419041 ]
- Bulaj G, Kortemme T, Goldenberg DP: Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry. 1998 Jun 23;37(25):8965-72. [9636038 ]
- Baker DH, Czarnecki-Maulden GL: Pharmacologic role of cysteine in ameliorating or exacerbating mineral toxicities. J Nutr. 1987 Jun;117(6):1003-10. [3298579 ]
- Sandmann J, Schwedhelm KS, Tsikas D: Specific transport of S-nitrosocysteine in human red blood cells: Implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Lett. 2005 Aug 1;579(19):4119-24. [16023102 ]
- Paivalainen S, Suokas M, Lahti O, Heape AM: Degraded myelin-associated glycoprotein (dMAG) formation from pure human brain myelin-associated glycoprotein (MAG) is not mediated by calpain or cathepsin L-like activities. J Neurochem. 2003 Feb;84(3):533-45. [12558973 ]
- Iyer S, Leonidas DD, Swaminathan GJ, Maglione D, Battisti M, Tucci M, Persico MG, Acharya KR: The crystal structure of human placenta growth factor-1 (PlGF-1), an angiogenic protein, at 2.0 A resolution. J Biol Chem. 2001 Apr 13;276(15):12153-61. Epub 2000 Nov 7. [11069911 ]
- Nishiya Y, Yoshida Y, Yoshimura M, Fukamachi H, Nakano Y: Homogeneous enzymatic assay for L-cysteine with betaC-S lyase. Biosci Biotechnol Biochem. 2005 Nov;69(11):2244-6. [16306712 ]
- Cynober LA: Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002 Sep;18(9):761-6. [12297216 ]
- Santamaria I, Velasco G, Cazorla M, Fueyo A, Campo E, Lopez-Otin C: Cathepsin L2, a novel human cysteine proteinase produced by breast and colorectal carcinomas. Cancer Res. 1998 Apr 15;58(8):1624-30. [9563472 ]
- Eriksson A, Tohonen V, Wedell A, Nordqvist K: Isolation of the human testatin gene and analysis in patients with abnormal gonadal development. Mol Hum Reprod. 2002 Jan;8(1):8-15. [11756564 ]
- Kaminska J, Wisniewska A, Koscielak J: Chemical modifications of alpha1,6-fucosyltransferase define amino acid residues of catalytic importance. Biochimie. 2003 Mar-Apr;85(3-4):303-10. [12770769 ]
- Li Y, Gamper N, Shapiro MS: Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent. J Neurosci. 2004 Jun 2;24(22):5079-90. [15175377 ]
- Lindzen M, Gottschalk KE, Fuzesi M, Garty H, Karlish SJ: Structural interactions between FXYD proteins and Na+,K+-ATPase: alpha/beta/FXYD subunit stoichiometry and cross-linking. J Biol Chem. 2006 Mar 3;281(9):5947-55. Epub 2005 Dec 21. [16373350 ]
- Norris FA, Wilson MP, Wallis TS, Galyov EE, Majerus PW: SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14057-9. [9826652 ]
- Kersemans V, Cornelissen B, Kersemans K, Bauwens M, Achten E, Dierckx RA, Mertens J, Slegers G: In vivo characterization of 123/125I-2-iodo-L-phenylalanine in an R1M rhabdomyosarcoma athymic mouse model as a potential tumor tracer for SPECT. J Nucl Med. 2005 Mar;46(3):532-9. [15750170 ]
- Foss CA, Mease RC, Fan H, Wang Y, Ravert HT, Dannals RF, Olszewski RT, Heston WD, Kozikowski AP, Pomper MG: Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin Cancer Res. 2005 Jun 1;11(11):4022-8. [15930336 ]
- Nicholson JK, O'Flynn MP, Sadler PJ, Macleod AF, Juul SM, Sonksen PH: Proton-nuclear-magnetic-resonance studies of serum, plasma and urine from fasting normal and diabetic subjects. Biochem J. 1984 Jan 15;217(2):365-75. [6696735 ]
- Kozaki K, Miyaishi O, Asai N, Iida K, Sakata K, Hayashi M, Nishida T, Matsuyama M, Shimizu S, Kaneda T, et al.: Tissue distribution of ERp61 and association of its increased expression with IgG production in hybridoma cells. Exp Cell Res. 1994 Aug;213(2):348-58. [8050492 ]
- Amberger VR, Hensel T, Ogata N, Schwab ME: Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998 Jan 1;58(1):149-58. [9426071 ]
- Zhang JT, Li QX, Wang D, Zhu ZL, Yang YH, Cui DS, Wang MW, Sun XF: Up-regulation of PINCH in the stroma of oral squamous cell carcinoma predicts nodal metastasis. Oncol Rep. 2005 Dec;14(6):1519-22. [16273248 ]
- Taveau M, Bourg N, Sillon G, Roudaut C, Bartoli M, Richard I: Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components. Mol Cell Biol. 2003 Dec;23(24):9127-35. [14645524 ]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R: Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003 Aug 20;23(20):7577-85. [12930796 ]
- Naisbitt DJ, Vilar FJ, Stalford AC, Wilkins EG, Pirmohamed M, Park BK: Plasma cysteine deficiency and decreased reduction of nitrososulfamethoxazole with HIV infection. AIDS Res Hum Retroviruses. 2000 Dec 10;16(18):1929-38. [11153075 ]
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [19212411 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|