| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-30 21:04:37 UTC |
|---|
| Update Date | 2014-12-24 20:26:52 UTC |
|---|
| Accession Number | T3D4562 |
|---|
| Identification |
|---|
| Common Name | Heroin |
|---|
| Class | Small Molecule |
|---|
| Description | A narcotic analgesic that may be habit-forming. It is a controlled substance (opium derivative) listed in the U.S. Code of Federal Regulations, Title 21 Parts 329.1, 1308.11 (1987). Sale is forbidden in the United States by Federal statute. (Merck Index, 11th ed) Internationally, heroin is controlled under Schedules I and IV of the Single Convention on Narcotic Drugs. It is illegal to manufacture, possess, or sell heroin in the United States and the UK. However, under the name diamorphine, heroin is a legal prescription drug in the United Kingdom. |
|---|
| Compound Type | - Amine
- Analgesic, Opioid
- Drug
- Ester
- Ether
- Narcotic
- Organic Compound
- Synthetic Compound
|
|---|
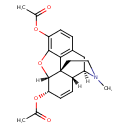
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (5alpha,6alpha)-7,8-Didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol diacetate (ester) | | 3,6-Diacetylmorphine | | 7,8-Dihydro-4,5-alpha-epoxy-17-methylmorphinan-3,6-alpha-diol diacetate | | diacetylmorphine | | diamorphine | | morphine diacetate | | O,O'-diacetylmorphine |
|
|---|
| Chemical Formula | C21H23NO5 |
|---|
| Average Molecular Mass | 369.411 g/mol |
|---|
| Monoisotopic Mass | 369.158 g/mol |
|---|
| CAS Registry Number | 561-27-3 |
|---|
| IUPAC Name | (1S,5R,13R,14S,17R)-14-(acetyloxy)-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10,15-tetraen-10-yl acetate |
|---|
| Traditional Name | (1S,5R,13R,14S,17R)-14-(acetyloxy)-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10,15-tetraen-10-yl acetate |
|---|
| SMILES | [H][C@@]12OC3=C(OC(C)=O)C=CC4=C3[C@@]11CCN(C)[C@]([H])(C4)[C@]1([H])C=C[C@]2([H])OC(C)=O |
|---|
| InChI Identifier | InChI=1S/C21H23NO5/c1-11(23)25-16-6-4-13-10-15-14-5-7-17(26-12(2)24)20-21(14,8-9-22(15)3)18(13)19(16)27-20/h4-7,14-15,17,20H,8-10H2,1-3H3/t14-,15+,17-,20-,21-/m0/s1 |
|---|
| InChI Key | InChIKey=GVGLGOZIDCSQPN-PVHGPHFFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as morphinans. These are polycyclic compounds with a four-ring skeleton with three condensed six-member rings forming a partially hydrogenated phenanthrene moiety, one of which is aromatic while the two others are alicyclic. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Morphinans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Morphinans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Morphinan
- Phenanthrene
- Tetralin
- Coumaran
- Alkyl aryl ether
- Aralkylamine
- Dicarboxylic acid or derivatives
- Piperidine
- Benzenoid
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Ether
- Carboxylic acid derivative
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 173 °C | | Boiling Point | 272-274 °C at 1.20E+01 mm Hg | | Solubility | 600 mg/L (at 25 °C) | | LogP | 1.58 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-016u-2079000000-b1a98da90b10b2f6fb14 | 2017-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0009000000-43bbf64aafa84c20e09c | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0009000000-d35dead9831fb810a273 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0039000000-3824b8beec641e65aced | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0303-0593000000-b8c7c4e8760df849eeb2 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-014l-0950000000-9cd1cf455c3a42888ee6 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-024i-0029000000-621a646ce875dfe29a86 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-1049000000-914d38bbfbcc120deba5 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-2091000000-4af238bd53e55b1a2237 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00or-2009000000-c77b2701df5a4dd2f03b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05r0-3029000000-ac3b8b35c5f87a9db00b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9052000000-69d8cee7b68a147f5c7f | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-016u-5955000000-17dd3ef75849a9fb63c3 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Bioavailability is less than 35%. |
|---|
| Mechanism of Toxicity | Heroin is a mu-opioid agonist. It acts on endogenous mu-opioid receptors that are spread in discrete packets throughout the brain, spinal cord and gut in almost all mammals. Heroin, along with other opioids, are agonists to four endogenous neurotransmitters. They are beta-endorphin, dynorphin, leu-enkephalin, and met-enkephalin. The body responds to heroin in the brain by reducing (and sometimes stopping) production of the endogenous opioids when heroin is present. Endorphins are regularly released in the brain and nerves, attenuating pain. Their other functions are still obscure, but are probably related to the effects produced by heroin besides analgesia (antitussin, anti-diarrheal). |
|---|
| Metabolism | Hepatic.
Route of Elimination: 90% renal as glucuronides, rest biliary
Half Life: <10 minutes |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used in the treatment of acute pain, myocardial infarction, acute pulmonary oedema, and chronic pain. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01452 |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 5462328 |
|---|
| ChEMBL ID | CHEMBL459324 |
|---|
| ChemSpider ID | 4575379 |
|---|
| KEGG ID | C06534 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 27808 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Heroin |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4562.pdf |
|---|
| General References | - Tschacher W, Haemmig R, Jacobshagen N: Time series modeling of heroin and morphine drug action. Psychopharmacology (Berl). 2003 Jan;165(2):188-93. Epub 2002 Oct 29. [12404073 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|