| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:18 UTC |
|---|
| Update Date | 2014-12-24 20:21:20 UTC |
|---|
| Accession Number | T3D0215 |
|---|
| Identification |
|---|
| Common Name | Chromic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Chromic acid generally refers to a collection of compounds generated by the acidification of solutions containing chromate and dichromate anions or the dissolving of chromium trioxide in sulfuric acid. Chromic acid contains hexavalent chromium. Hexavalent chromium refers to chromium in the +6 oxidation state, and is more toxic than other oxidation states of the chromium atom because of its greater ability to enter cells and a higher redox potential. (9) Molecular chromic acid, H2CrO4, has much in common with sulfuric acid, H2SO4 as both are classified as strong acids. Chromic acid was widely used in the instrument repair industry, due to its ability to "brighten" raw brass. A chromic acid dip leaves behind a bright yellow patina on the brass. Due to growing health and environmental concerns, many have discontinued use of this chemical in their repair shops. Most chromic acid sold or available as a 10% aqueous solution. |
|---|
| Compound Type | - Chromium Compound
- Industrial/Workplace Toxin
- Inorganic Compound
- Pollutant
- Synthetic Compound
|
|---|
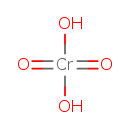
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Chromate | | Chromic Acid | | Chromium hydroxide oxide | | Dihydrogen(tetraaoxidochromate) | | Dihydroxidodioxidochromium | | H2CrO4 | | Tetraoxochromic acid | | [CrO2(OH)2] |
|
|---|
| Chemical Formula | CrH2O4 |
|---|
| Average Molecular Mass | 118.010 g/mol |
|---|
| Monoisotopic Mass | 117.936 g/mol |
|---|
| CAS Registry Number | 7738-94-5 |
|---|
| IUPAC Name | dioxochromiumdiol |
|---|
| Traditional Name | chromic acid |
|---|
| SMILES | O[Cr](O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/Cr.2H2O.2O/h;2*1H2;;/q+2;;;;/p-2 |
|---|
| InChI Key | InChIKey=KRVSOGSZCMJSLX-UHFFFAOYSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as miscellaneous chromates. These are inorganic compounds in which the largest metallic oxoanion is chromate, to which either no atom or a non metal atom is bonded. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Miscellaneous mixed metal/non-metals |
|---|
| Sub Class | Miscellaneous metallic oxoanionic compounds |
|---|
| Direct Parent | Miscellaneous chromates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chromate
- Inorganic oxide
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid or Liquid |
|---|
| Appearance | Red powder; Orange liquid (acid) |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 197°C (solid) | | Boiling Point | 100°C (acid); 250°C (solid) | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-f339e8b185b6511879a0 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-0ffb6d3c099fe25d4838 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-1900000000-0652730d77e7f81111f0 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-2b794de366bf8de77cf3 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1900000000-5a683e4c85e8b7dc2182 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0900000000-82a448a699a8ecda2657 | 2019-02-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (8) ; inhalation (8) ; dermal (8) |
|---|
| Mechanism of Toxicity | Chromic acid is highly corrosive and strongly oxidative. Many strong acids cause tissue burns through the denaturation of proteins and partial hydrolysis of proteins. Most proteins denature at pH values of less than 3-4. The large-scale denaturation of proteins, de-esterification of lipids and subsequent desiccation of tissues leads to chemical burns. . Symptoms include itching, bleaching or darkening of skin or tissues, blistering and burning sensations. Chromic acid also denatures proteins through inserting oxygen atoms into protein side chains. Chromic acid is also a source of chromium and especially hexavalent chromium. Hexavalent chromium compounds (including chromium trioxide, chromic acids, chromates, chlorochromates) are toxic and carcinogenic. For this reason, chromic acid oxidation is not used on an industrial scale except in the aerospace industry. Hexavalent chromium's carcinogenic effects are caused by its metabolites, pentavalent and trivalent chromium. The DNA damage may be caused by hydroxyl radicals produced during reoxidation of pentavalent chromium by hydrogen peroxide molecules present in the cell. Trivalent chromium may also form complexes with peptides, proteins, and DNA, resulting in DNA-protein crosslinks, DNA strand breaks, DNA-DNA interstrand crosslinks, chromium-DNA adducts, chromosomal aberrations and alterations in cellular signaling pathways. It has been shown to induce carcinogenesis by overstimulating cellular regulatory pathways and increasing peroxide levels by activating certain mitogen-activated protein kinases. It can also cause transcriptional repression by cross-linking histone deacetylase 1-DNA methyltransferase 1 complexes to CYP1A1 promoter chromatin, inhibiting histone modification. Chromium may increase its own toxicity by modifying metal regulatory transcription factor 1, causing the inhibition of zinc-induced metallothionein transcription. (1, 8, 2, 3, 4) |
|---|
| Metabolism | Skin contact with chromic acid can cause redness, pain, and severe skin burns. Chromic acid may cause severe burns to the eye and permanent eye damage. Severe and rapid corrosive burns of the mouth, gullet and gastrointestinal tract will result if chromic acid is swallowed. Symptoms include burning, choking, nausea, vomiting and severe pain. Chronic exposure to low levels of chromic acid can lead to chronic exposure to hexavalent chromium. Hexavalent chromium is a known carcinogen. Chronic inhalation especially has been linked to lung cancer. Hexavalent chromium is also known to cause reproductive and developmental defects. (1) |
|---|
| Toxicity Values | LD50: 330 mg/kg (Oral, Dog) (6) |
|---|
| Lethal Dose | 1 to 3 grams for an adult human (hexavalent chromium). (5) |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (12) |
|---|
| Uses/Sources | Chromic acid is an intermediate in chromium plating, and is also used in ceramic glazes, and colored glass. (10) |
|---|
| Minimum Risk Level | Intermediate Oral: 0.005 mg/kg/day (11)
Chronic Oral: 0.001 mg/kg/day (11) |
|---|
| Health Effects | Skin contact with chromic acid can cause redness, pain, and severe skin burns. Chromic acid may cause severe burns to the eye and permanent eye damage. Severe and rapid corrosive burns of the mouth, gullet and gastrointestinal tract will result if chromic acid is swallowed. Symptoms include burning, choking, nausea, vomiting and severe pain. Chronic exposure to low levels of chromic acid can lead to chronic exposure to hexavalent chromium. Hexavalent chromium is a known carcinogen. Chronic inhalation especially has been linked to lung cancer. Hexavalent chromium is also known to cause reproductive and developmental defects. (1) |
|---|
| Symptoms | Skin contact can cause redness, pain, and severe skin burns. Chromic acid may cause severe burns to the eye and permanent eye damage. Severe and rapid corrosive burns of the mouth, gullet and gastrointestinal tract will result if chromic acid is swallowed. Symptoms include burning, choking, nausea, vomiting and severe pain. |
|---|
| Treatment | The mainstay of treatment of any acid burn is copious irrigation with large amounts of tap water. To be most effective, treatment should be started immediately after exposure, preferably before arrival in the emergency department. Remove any contaminated clothing. Do not attempt to neutralize the burn with weak reciprocal chemicals (i.e. alkali for acid burns), because the heat generated from the chemical reaction may cause severe thermal injury. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 24425 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 22834 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 33143 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Chromic acid |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 7935 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0215.pdf |
|---|
| General References | - Salnikow K, Zhitkovich A: Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008 Jan;21(1):28-44. Epub 2007 Oct 30. [17970581 ]
- Kim G, Yurkow EJ: Chromium induces a persistent activation of mitogen-activated protein kinases by a redox-sensitive mechanism in H4 rat hepatoma cells. Cancer Res. 1996 May 1;56(9):2045-51. [8616849 ]
- Schnekenburger M, Talaska G, Puga A: Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007 Oct;27(20):7089-101. Epub 2007 Aug 6. [17682057 ]
- Kimura T: [Molecular mechanism involved in chromium(VI) toxicity]. Yakugaku Zasshi. 2007 Dec;127(12):1957-65. [18057785 ]
- Barceloux DG: Chromium. J Toxicol Clin Toxicol. 1999;37(2):173-94. [10382554 ]
- Folli C, Viglione S, Busconi M, Berni R: Biochemical basis for retinol deficiency induced by the I41N and G75D mutations in human plasma retinol-binding protein. Biochem Biophys Res Commun. 2005 Nov 4;336(4):1017-22. [16157297 ]
- Sax NI (1987). Dangerous Properties of Industrial Materials Reports. New York: Van Nostrand Rheinhold.
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for chromium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Chromic acid. Last Updated 11 March 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1997). Toxicological profile for tetrachloroethylene (PERC) . U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|