Doxepin (T3D2993)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:28:26 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:55 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2993 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Doxepin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Doxepin hydrochloride is a dibenzoxepin-derivative tricyclic antidepressant (TCA). TCAs are structurally similar to phenothiazines. They contain a tricyclic ring system with an alkyl amine substituent on the central ring. In non-depressed individuals, doxepin does not affect mood or arousal, but may cause sedation. In depressed individuals, doxepin exerts a positive effect on mood. TCAs are potent inhibitors of serotonin and norepinephrine reuptake. Tertiary amine TCAs, such as doxepin and amitriptyline, are more potent inhibitors of serotonin reuptake than secondary amine TCAs, such as nortriptyline and desipramine. TCAs also down-regulate cerebral cortical β-adrenergic receptors and sensitize post-synaptic serotonergic receptors with chronic use. The antidepressant effects of TCAs are thought to be due to an overall increase in serotonergic neurotransmission. TCAs also block histamine H1 receptors, α1-adrenergic receptors and muscarinic receptors, which accounts for their sedative, hypotensive and anticholinergic effects (e.g. blurred vision, dry mouth, constipation, urinary retention), respectively. Doxepin has less sedative and anticholinergic effects than amitriptyline. See toxicity section below for a complete listing of side effects. Doxepin may be used to treat depression and insomnia. Unlabeled indications include chronic and neuropathic pain, and anxiety. Doxepin may also be used as a second line agent to treat idiopathic urticaria. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
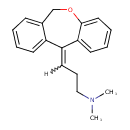
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C19H21NO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 279.376 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 279.162 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 1668-19-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | dimethyl(3-{9-oxatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,11,13-hexaen-2-ylidene}propyl)amine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | doxepin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [H]\C(CCN(C)C)=C1/C2=CC=CC=C2COC2=CC=CC=C12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=ODQWQRRAPPTVAG-BOPFTXTBSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as dibenzoxepines. Dibenzoxepines are compounds containing a dibenzoxepine moiety, which consists of two benzene connected by an oxazepine ring. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzoxepines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Dibenzoxepines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Dibenzoxepines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Well-absorbed from the GI tract. Peak plasma concentrations occur within 2 hours of oral administration. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | The mechanism of action of doxepin is not completely understood. It is thought that like amitriptyline, doxepin enhances the actions of norepinephrine and serotonin by blocking their reuptake at the neuronal membrane. However, doxepin weakly inhibits the reuptake of dopamine. Doxepin may also act on histamine H1-receptors, resulting in sedative effects, and β-adrenergic receptors. It is also an antagonist of 5-hydroxytryptamine (serotonin) receptors, alpha-1 adrenergic receptor, and muscarinic cholinergic receptors. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Extensively metabolized in the liver via the same pathways as other TCAs. N-demethylation produces an active metabolite, N-desmethyldoxepin. CYP2D6 specifically hydroxylates the E-isomer. Half Life: 6 - 24.5 hours | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 26 mg/kg (Intravenous, Mouse) (1) LD50: 16 mg/kg (Intravenous, Rat) (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Doxepin is used for the treatment of depression, insomina, alcohol and drug withdrawal, gastrointestinal ulceration and other GI-problems. It can also be used for chronic urticaria and in the management of pain. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | hypotension, postural collapse (if patient arises too fast from lying/sitting position to standing), arrhythmias (sinus-tachycardia, bradycardia, av-blockade). Allergic/toxic effetcs also include skin rash, photosensitivity, liver damage of the cholostatic type (rarely), hepatitis (extremely rare), leuko- or thrombopenia (rarely), agranulocytosis (very rarely), hypoplastic anemia (rarely). [Wikipedia] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is strongly advised. If signs of toxicity occur at any time during this period, extended monitoring is recommended. (8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB01142 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB15273 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 667468 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL1628227 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 580850 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C06971 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 4710 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Doxepin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | D7V | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Doxepin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Luigi Schioppi, Brian Talmadge Dorsey, Michael Skinner, John Carter, Robert Mansbach, Philip Jochelson, Roberta L. Rogowski, Cara Casseday, Meredith Perry, Bryan Knox, “LOW-DOSE DOXEPIN FORMULATIONS AND METHODS OF MAKING AND USING THE SAME.” U.S. Patent US20090074862, issued March 19, 2009. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Histamine receptor activity
- Specific Function:
- In peripheral tissues, the H1 subclass of histamine receptors mediates the contraction of smooth muscles, increase in capillary permeability due to contraction of terminal venules, and catecholamine release from adrenal medulla, as well as mediating neurotransmission in the central nervous system.
- Gene Name:
- HRH1
- Uniprot ID:
- P35367
- Molecular Weight:
- 55783.61 Da
References
- Tashiro M, Sakurada Y, Iwabuchi K, Mochizuki H, Kato M, Aoki M, Funaki Y, Itoh M, Iwata R, Wong DF, Yanai K: Central effects of fexofenadine and cetirizine: measurement of psychomotor performance, subjective sleepiness, and brain histamine H1-receptor occupancy using 11C-doxepin positron emission tomography. J Clin Pharmacol. 2004 Aug;44(8):890-900. [15286093 ]
- Richelson E, Nelson A: Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984 Jul;230(1):94-102. [6086881 ]
- Tran VT, Lebovitz R, Toll L, Snyder SH: [3H]doxepin interactions with histamine H1-receptors and other sites in guinea pig and rat brain homogenates. Eur J Pharmacol. 1981 Apr 9;70(4):501-9. [7238574 ]
- Kano M, Fukudo S, Tashiro A, Utsumi A, Tamura D, Itoh M, Iwata R, Tashiro M, Mochizuki H, Funaki Y, Kato M, Hongo M, Yanai K: Decreased histamine H1 receptor binding in the brain of depressed patients. Eur J Neurosci. 2004 Aug;20(3):803-10. [15255990 ]
- Claro E, Arbones L, Garcia A, Picatoste F: Phosphoinositide hydrolysis mediated by histamine H1-receptors in rat brain cortex. Eur J Pharmacol. 1986 Apr 16;123(2):187-96. [3011460 ]
- General Function:
- Phosphatidylinositol phospholipase c activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM1
- Uniprot ID:
- P11229
- Molecular Weight:
- 51420.375 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Stahl SM: Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008 Dec;13(12):1027-38. [19179941 ]
- Ehlert FJ, Delen FM, Yun SH, Liem HA: The interaction of amitriptyline, doxepin, imipramine and their N-methyl quaternary ammonium derivatives with subtypes of muscarinic receptors in brain and heart. J Pharmacol Exp Ther. 1990 Apr;253(1):13-9. [2329499 ]
- Figueiredo A, Ribeiro CA, Goncalo M, Almeida L, Poiares-Baptista A, Teixeira F: Mechanism of action of doxepin in the treatment of chronic urticaria. Fundam Clin Pharmacol. 1990;4(2):147-58. [2141000 ]
- General Function:
- Virus receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including mescaline, psilocybin, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates phospholipase C and a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and promotes the release of Ca(2+) ions from intracellular stores. Affects neural activity, perception, cognition and mood. Plays a role in the regulation of behavior, including responses to anxiogenic situations and psychoactive substances. Plays a role in intestinal smooth muscle contraction, and may play a role in arterial vasoconstriction.(Microbial infection) Acts as a receptor for human JC polyomavirus/JCPyV.
- Gene Name:
- HTR2A
- Uniprot ID:
- P28223
- Molecular Weight:
- 52602.58 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Maj J, Gancarczyk L, Gorszczyk L, Rawlow A: Doxepin as a blocker of central serotonin receptors. Pharmakopsychiatr Neuropsychopharmakol. 1977 Dec;10(6):318-24. [309138 ]
- Stahl SM: Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008 Dec;13(12):1027-38. [19179941 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- This alpha-adrenergic receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Its effect is mediated by G(q) and G(11) proteins. Nuclear ADRA1A-ADRA1B heterooligomers regulate phenylephrine(PE)-stimulated ERK signaling in cardiac myocytes.
- Gene Name:
- ADRA1A
- Uniprot ID:
- P35348
- Molecular Weight:
- 51486.005 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Stahl SM: Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008 Dec;13(12):1027-38. [19179941 ]
- Richelson E, Nelson A: Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984 Jul;230(1):94-102. [6086881 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- This alpha-adrenergic receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Its effect is mediated by G(q) and G(11) proteins. Nuclear ADRA1A-ADRA1B heterooligomers regulate phenylephrine (PE)-stimulated ERK signaling in cardiac myocytes.
- Gene Name:
- ADRA1B
- Uniprot ID:
- P35368
- Molecular Weight:
- 56835.375 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Stahl SM: Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008 Dec;13(12):1027-38. [19179941 ]
- Richelson E, Nelson A: Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984 Jul;230(1):94-102. [6086881 ]
- General Function:
- Alpha1-adrenergic receptor activity
- Specific Function:
- This alpha-adrenergic receptor mediates its effect through the influx of extracellular calcium.
- Gene Name:
- ADRA1D
- Uniprot ID:
- P25100
- Molecular Weight:
- 60462.205 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Stahl SM: Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008 Dec;13(12):1027-38. [19179941 ]
- Richelson E, Nelson A: Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984 Jul;230(1):94-102. [6086881 ]
- General Function:
- G-protein coupled acetylcholine receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is adenylate cyclase inhibition. Signaling promotes phospholipase C activity, leading to the release of inositol trisphosphate (IP3); this then triggers calcium ion release into the cytosol.
- Gene Name:
- CHRM2
- Uniprot ID:
- P08172
- Molecular Weight:
- 51714.605 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Ehlert FJ, Delen FM, Yun SH, Liem HA: The interaction of amitriptyline, doxepin, imipramine and their N-methyl quaternary ammonium derivatives with subtypes of muscarinic receptors in brain and heart. J Pharmacol Exp Ther. 1990 Apr;253(1):13-9. [2329499 ]
- Figueiredo A, Ribeiro CA, Goncalo M, Almeida L, Poiares-Baptista A, Teixeira F: Mechanism of action of doxepin in the treatment of chronic urticaria. Fundam Clin Pharmacol. 1990;4(2):147-58. [2141000 ]
- General Function:
- Receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM3
- Uniprot ID:
- P20309
- Molecular Weight:
- 66127.445 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Ehlert FJ, Delen FM, Yun SH, Liem HA: The interaction of amitriptyline, doxepin, imipramine and their N-methyl quaternary ammonium derivatives with subtypes of muscarinic receptors in brain and heart. J Pharmacol Exp Ther. 1990 Apr;253(1):13-9. [2329499 ]
- Figueiredo A, Ribeiro CA, Goncalo M, Almeida L, Poiares-Baptista A, Teixeira F: Mechanism of action of doxepin in the treatment of chronic urticaria. Fundam Clin Pharmacol. 1990;4(2):147-58. [2141000 ]
- General Function:
- Guanyl-nucleotide exchange factor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is inhibition of adenylate cyclase.
- Gene Name:
- CHRM4
- Uniprot ID:
- P08173
- Molecular Weight:
- 53048.65 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Ehlert FJ, Delen FM, Yun SH, Liem HA: The interaction of amitriptyline, doxepin, imipramine and their N-methyl quaternary ammonium derivatives with subtypes of muscarinic receptors in brain and heart. J Pharmacol Exp Ther. 1990 Apr;253(1):13-9. [2329499 ]
- Figueiredo A, Ribeiro CA, Goncalo M, Almeida L, Poiares-Baptista A, Teixeira F: Mechanism of action of doxepin in the treatment of chronic urticaria. Fundam Clin Pharmacol. 1990;4(2):147-58. [2141000 ]
- General Function:
- Phosphatidylinositol phospholipase c activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM5
- Uniprot ID:
- P08912
- Molecular Weight:
- 60073.205 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Ehlert FJ, Delen FM, Yun SH, Liem HA: The interaction of amitriptyline, doxepin, imipramine and their N-methyl quaternary ammonium derivatives with subtypes of muscarinic receptors in brain and heart. J Pharmacol Exp Ther. 1990 Apr;253(1):13-9. [2329499 ]
- Figueiredo A, Ribeiro CA, Goncalo M, Almeida L, Poiares-Baptista A, Teixeira F: Mechanism of action of doxepin in the treatment of chronic urticaria. Fundam Clin Pharmacol. 1990;4(2):147-58. [2141000 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Gene Name:
- HTR1A
- Uniprot ID:
- P08908
- Molecular Weight:
- 46106.335 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Maj J, Gancarczyk L, Gorszczyk L, Rawlow A: Doxepin as a blocker of central serotonin receptors. Pharmakopsychiatr Neuropsychopharmakol. 1977 Dec;10(6):318-24. [309138 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various ergot alkaloid derivatives and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of dopamine and 5-hydroxytryptamine release, 5-hydroxytryptamine uptake and in the regulation of extracellular dopamine and 5-hydroxytryptamine levels, and thereby affects neural activity. May play a role in the perception of pain. Plays a role in the regulation of behavior, including impulsive behavior. Required for normal proliferation of embryonic cardiac myocytes and normal heart development. Protects cardiomyocytes against apoptosis. Plays a role in the adaptation of pulmonary arteries to chronic hypoxia. Plays a role in vasoconstriction. Required for normal osteoblast function and proliferation, and for maintaining normal bone density. Required for normal proliferation of the interstitial cells of Cajal in the intestine.
- Gene Name:
- HTR2B
- Uniprot ID:
- P41595
- Molecular Weight:
- 54297.41 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Maj J, Gancarczyk L, Gorszczyk L, Rawlow A: Doxepin as a blocker of central serotonin receptors. Pharmakopsychiatr Neuropsychopharmakol. 1977 Dec;10(6):318-24. [309138 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including ergot alkaloid derivatives, 1-2,5,-dimethoxy-4-iodophenyl-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Regulates neuronal activity via the activation of short transient receptor potential calcium channels in the brain, and thereby modulates the activation of pro-opiomelacortin neurons and the release of CRH that then regulates the release of corticosterone. Plays a role in the regulation of appetite and eating behavior, responses to anxiogenic stimuli and stress. Plays a role in insulin sensitivity and glucose homeostasis.
- Gene Name:
- HTR2C
- Uniprot ID:
- P28335
- Molecular Weight:
- 51820.705 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Maj J, Gancarczyk L, Gorszczyk L, Rawlow A: Doxepin as a blocker of central serotonin receptors. Pharmakopsychiatr Neuropsychopharmakol. 1977 Dec;10(6):318-24. [309138 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which inhibit adenylyl cyclase.
- Gene Name:
- DRD2
- Uniprot ID:
- P14416
- Molecular Weight:
- 50618.91 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- Richelson E, Nelson A: Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984 Jul;230(1):94-102. [6086881 ]
- General Function:
- Norepinephrine:sodium symporter activity
- Specific Function:
- Amine transporter. Terminates the action of noradrenaline by its high affinity sodium-dependent reuptake into presynaptic terminals.
- Gene Name:
- SLC6A2
- Uniprot ID:
- P23975
- Molecular Weight:
- 69331.42 Da
References
- General Function:
- Serotonin:sodium symporter activity
- Specific Function:
- Serotonin transporter whose primary function in the central nervous system involves the regulation of serotonergic signaling via transport of serotonin molecules from the synaptic cleft back into the pre-synaptic terminal for re-utilization. Plays a key role in mediating regulation of the availability of serotonin to other receptors of serotonergic systems. Terminates the action of serotonin and recycles it in a sodium-dependent manner.
- Gene Name:
- SLC6A4
- Uniprot ID:
- P31645
- Molecular Weight:
- 70324.165 Da
References
- General Function:
- Thioesterase binding
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins. The rank order of potency for agonists of this receptor is oxymetazoline > clonidine > epinephrine > norepinephrine > phenylephrine > dopamine > p-synephrine > p-tyramine > serotonin = p-octopamine. For antagonists, the rank order is yohimbine > phentolamine = mianserine > chlorpromazine = spiperone = prazosin > propanolol > alprenolol = pindolol.
- Gene Name:
- ADRA2A
- Uniprot ID:
- P08913
- Molecular Weight:
- 48956.275 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- General Function:
- Epinephrine binding
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins. The rank order of potency for agonists of this receptor is clonidine > norepinephrine > epinephrine = oxymetazoline > dopamine > p-tyramine = phenylephrine > serotonin > p-synephrine / p-octopamine. For antagonists, the rank order is yohimbine > chlorpromazine > phentolamine > mianserine > spiperone > prazosin > alprenolol > propanolol > pindolol.
- Gene Name:
- ADRA2B
- Uniprot ID:
- P18089
- Molecular Weight:
- 49565.8 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins.
- Gene Name:
- ADRA2C
- Uniprot ID:
- P18825
- Molecular Weight:
- 49521.585 Da
References
- Cusack B, Nelson A, Richelson E: Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl). 1994 May;114(4):559-65. [7855217 ]
- General Function:
- Histamine receptor activity
- Specific Function:
- The H2 subclass of histamine receptors mediates gastric acid secretion. Also appears to regulate gastrointestinal motility and intestinal secretion. Possible role in regulating cell growth and differentiation. The activity of this receptor is mediated by G proteins which activate adenylyl cyclase and, through a separate G protein-dependent mechanism, the phosphoinositide/protein kinase (PKC) signaling pathway (By similarity).
- Gene Name:
- HRH2
- Uniprot ID:
- P25021
- Molecular Weight:
- 40097.65 Da
References
- Beil W, Hannemann H, Sewing KF: Interaction of antidepressants and neuroleptics with histamine stimulated parietal cell adenylate cyclase and H+ secretion. Pharmacology. 1988;36(3):198-203. [2897127 ]
- General Function:
- Voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization
- Specific Function:
- Pore-forming (alpha) subunit of voltage-gated inwardly rectifying potassium channel. Channel properties are modulated by cAMP and subunit assembly. Mediates the rapidly activating component of the delayed rectifying potassium current in heart (IKr). Isoforms USO have no channel activity by themself, but modulates channel characteristics by forming heterotetramers with other isoforms which are retained intracellularly and undergo ubiquitin-dependent degradation.
- Gene Name:
- KCNH2
- Uniprot ID:
- Q12809
- Molecular Weight:
- 126653.52 Da
References
- Duncan RS, McPate MJ, Ridley JM, Gao Z, James AF, Leishman DJ, Leaney JL, Witchel HJ, Hancox JC: Inhibition of the HERG potassium channel by the tricyclic antidepressant doxepin. Biochem Pharmacol. 2007 Aug 1;74(3):425-37. Epub 2007 May 3. [17560554 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that stimulate adenylate cyclase. It has a high affinity for tricyclic psychotropic drugs (By similarity). Controls pyramidal neurons migration during corticogenesis, through the regulation of CDK5 activity (By similarity). Is an activator of TOR signaling (PubMed:23027611).
- Gene Name:
- HTR6
- Uniprot ID:
- P50406
- Molecular Weight:
- 46953.625 Da
- General Function:
- Histamine receptor activity
- Specific Function:
- The H4 subclass of histamine receptors could mediate the histamine signals in peripheral tissues. Displays a significant level of constitutive activity (spontaneous activity in the absence of agonist).
- Gene Name:
- HRH4
- Uniprot ID:
- Q9H3N8
- Molecular Weight:
- 44495.375 Da