Halothane (T3D3001)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:28:29 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:55 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D3001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Halothane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | A nonflammable, halogenated, hydrocarbon anesthetic that provides relatively rapid induction with little or no excitement. Analgesia may not be adequate. nitrous oxide is often given concomitantly. Because halothane may not produce sufficient muscle relaxation, supplemental neuromuscular blocking agents may be required. (From AMA Drug Evaluations Annual, 1994, p178) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
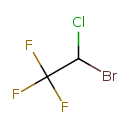
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C2HBrClF3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 197.382 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 195.890 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 151-67-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 2-bromo-2-chloro-1,1,1-trifluoroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | halothane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | FC(F)(F)C(Cl)Br | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1/C2HBrClF3/c3-1(4)2(5,6)7/h1H | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=BCQZXOMGPXTTIC-UHFFFAOYNA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as organofluorides. Organofluorides are compounds containing a chemical bond between a carbon atom and a fluorine atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organohalogen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organofluorides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Organofluorides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | inhalation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Halothane causes general anaethesia due to its actions on multiple ion channels, which ultimately depresses nerve conduction, breathing, cardiac contractility. Its immobilizing effects have been attributed to its binding to potassium channels in cholinergic neurons. Halothane's effect are also likely due to binding to NMDA and calcium channels, causing hyperpolarization. Halothane induces a reduction in junctional conductance by decreasing gap junction channel opening times and increasing gap junction channel closing times. Halothane also activates calcium dependent ATPase in the sarcoplasmic reticulum by increasing the fluidity of the lipid membrane. Also appears to bind the D subunit of ATP synthase and NADH dehydogenase. Halothane also binds to the GABA receptor, the large conductance Ca2+ activated potassium channel, the glutamate receptor and the glycine receptor. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Halothane is metabolized in the liver, primarily by CYP2E1, and to a lesser extent by CYP3A4 and CYP2A6. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For the induction and maintenance of general anesthesia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Damage or injury to the liver. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Asymptomatic - mild liver damage, nausea, vomitting, abdominal pain, loss of appetite. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | In the event of overdosage, or what may appear to be overdosage, drug administration should be stopped, and assisted or controlled ventilation with pure oxygen initiated. There is no specific antidote. Treatment should be aimed at maintaining respiratory function (by moving the patient to fresh air or inserting an emergency airway with respiratory support) and cardiovascular function. Cases of internal ingestion must be treated symptomatically. (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB01159 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB15290 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 3562 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL931 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 3441 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C07515 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 5615 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Halothane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Halothane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | U.S. Patents 2,849,502, 2,921,098, 2,959,624, 3,082,263. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Toxic substance binding
- Specific Function:
- Serum albumin, the main protein of plasma, has a good binding capacity for water, Ca(2+), Na(+), K(+), fatty acids, hormones, bilirubin and drugs. Its main function is the regulation of the colloidal osmotic pressure of blood. Major zinc transporter in plasma, typically binds about 80% of all plasma zinc.
- Gene Name:
- ALB
- Uniprot ID:
- P02768
- Molecular Weight:
- 69365.94 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 2900 uM | Not Available | BindingDB 50112212 |
References
- Chan K, Meng QC, Johansson JS, Eckenhoff RG: Low-affinity analytical chromatography for measuring inhaled anesthetic binding to isolated proteins. Anal Biochem. 2002 Feb 15;301(2):308-13. [11814301 ]
- Solt K, Johansson JS: Binding of the active metabolite of chloral hydrate, 2,2,2-trichloroethanol, to serum albumin demonstrated using tryptophan fluorescence quenching. Pharmacology. 2002;64(3):152-9. [11834892 ]
- Liu R, Pidikiti R, Ha CE, Petersen CE, Bhagavan NV, Eckenhoff RG: The role of electrostatic interactions in human serum albumin binding and stabilization by halothane. J Biol Chem. 2002 Sep 27;277(39):36373-9. Epub 2002 Jul 12. [12118010 ]
- Liu R, Meng Q, Xi J, Yang J, Ha CE, Bhagavan NV, Eckenhoff RG: Comparative binding character of two general anaesthetics for sites on human serum albumin. Biochem J. 2004 May 15;380(Pt 1):147-52. [14759223 ]
- Streiff JH, Juranic NO, Macura SI, Warner DO, Jones KA, Perkins WJ: Saturation transfer difference nuclear magnetic resonance spectroscopy as a method for screening proteins for anesthetic binding. Mol Pharmacol. 2004 Oct;66(4):929-35. [15385643 ]
- Eckenhoff RG, Knoll FJ, Greenblatt EP, Dailey WP: Halogenated diazirines as photolabel mimics of the inhaled haloalkane anesthetics. J Med Chem. 2002 Apr 25;45(9):1879-86. [11960499 ]
- General Function:
- Signal transducer activity
- Specific Function:
- Guanine nucleotide-binding proteins (G proteins) are involved as a modulator or transducer in various transmembrane signaling systems. The beta and gamma chains are required for the GTPase activity, for replacement of GDP by GTP, and for G protein-effector interaction (By similarity).
- Gene Name:
- GNG2
- Uniprot ID:
- P59768
- Molecular Weight:
- 7850.03 Da
References
- Ishizawa Y, Sharp R, Liebman PA, Eckenhoff RG: Halothane binding to a G protein coupled receptor in retinal membranes by photoaffinity labeling. Biochemistry. 2000 Jul 25;39(29):8497-502. [10913255 ]
- Milovic S, Steinecker-Frohnwieser B, Schreibmayer W, Weigl LG: The sensitivity of G protein-activated K+ channels toward halothane is essentially determined by the C terminus. J Biol Chem. 2004 Aug 13;279(33):34240-9. Epub 2004 Jun 2. [15175324 ]
- Zang WJ, Yu XJ, Zang YM: [Effect of halothane on the muscarinic potassium current of the heart]. Sheng Li Xue Bao. 2000 Apr;52(2):175-8. [11961592 ]
- Yoshimura H, Jones KA, Perkins WJ, Warner DO: Dual effects of hexanol and halothane on the regulation of calcium sensitivity in airway smooth muscle. Anesthesiology. 2003 Apr;98(4):871-80. [12657848 ]
- Streiff J, Jones K, Perkins WJ, Warner DO, Jones KA: Effect of halothane on the guanosine 5' triphosphate binding activity of G-protein alphai subunits. Anesthesiology. 2003 Jul;99(1):105-11. [12826849 ]
- General Function:
- G-protein activated inward rectifier potassium channel activity
- Specific Function:
- This potassium channel is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This receptor plays a crucial role in regulating the heartbeat.
- Gene Name:
- KCNJ3
- Uniprot ID:
- P48549
- Molecular Weight:
- 56602.84 Da
References
- Milovic S, Steinecker-Frohnwieser B, Schreibmayer W, Weigl LG: The sensitivity of G protein-activated K+ channels toward halothane is essentially determined by the C terminus. J Biol Chem. 2004 Aug 13;279(33):34240-9. Epub 2004 Jun 2. [15175324 ]
- Weigl LG, Schreibmayer W: G protein-gated inwardly rectifying potassium channels are targets for volatile anesthetics. Mol Pharmacol. 2001 Aug;60(2):282-9. [11455015 ]
- Yamakura T, Lewohl JM, Harris RA: Differential effects of general anesthetics on G protein-coupled inwardly rectifying and other potassium channels. Anesthesiology. 2001 Jul;95(1):144-53. [11465552 ]
- General Function:
- Vasopressin receptor activity
- Specific Function:
- G-protein coupled receptor for neuropeptide S (NPS) (PubMed:16790440). Promotes mobilization of intracellular Ca(2+) stores (PubMed:16790440). Inhibits cell growth in response to NPS binding (PubMed:15947423). Involved in pathogenesis of asthma and other IgE-mediated diseases.
- Gene Name:
- NPSR1
- Uniprot ID:
- Q6W5P4
- Molecular Weight:
- 42686.28 Da
References
- Ishizawa Y, Sharp R, Liebman PA, Eckenhoff RG: Halothane binding to a G protein coupled receptor in retinal membranes by photoaffinity labeling. Biochemistry. 2000 Jul 25;39(29):8497-502. [10913255 ]
- Streiff J, Jones K, Perkins WJ, Warner DO, Jones KA: Effect of halothane on the guanosine 5' triphosphate binding activity of G-protein alphai subunits. Anesthesiology. 2003 Jul;99(1):105-11. [12826849 ]
- Ishizawa Y, Pidikiti R, Liebman PA, Eckenhoff RG: G protein-coupled receptors as direct targets of inhaled anesthetics. Mol Pharmacol. 2002 May;61(5):945-52. [11961111 ]
- General Function:
- Transporter activity
- Specific Function:
- Mitochondrial membrane ATP synthase (F(1)F(0) ATP synthase or Complex V) produces ATP from ADP in the presence of a proton gradient across the membrane which is generated by electron transport complexes of the respiratory chain. F-type ATPases consist of two structural domains, F(1) - containing the extramembraneous catalytic core, and F(0) - containing the membrane proton channel, linked together by a central stalk and a peripheral stalk. During catalysis, ATP turnover in the catalytic domain of F(1) is coupled via a rotary mechanism of the central stalk subunits to proton translocation. Part of the complex F(1) domain and of the central stalk which is part of the complex rotary element. Rotation of the central stalk against the surrounding alpha(3)beta(3) subunits leads to hydrolysis of ATP in three separate catalytic sites on the beta subunits.
- Gene Name:
- ATP5D
- Uniprot ID:
- P30049
- Molecular Weight:
- 17489.755 Da
References
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Potassium channel activated by both membrane depolarization or increase in cytosolic Ca(2+) that mediates export of K(+). It is also activated by the concentration of cytosolic Mg(2+). Its activation dampens the excitatory events that elevate the cytosolic Ca(2+) concentration and/or depolarize the cell membrane. It therefore contributes to repolarization of the membrane potential. Plays a key role in controlling excitability in a number of systems, such as regulation of the contraction of smooth muscle, the tuning of hair cells in the cochlea, regulation of transmitter release, and innate immunity. In smooth muscles, its activation by high level of Ca(2+), caused by ryanodine receptors in the sarcoplasmic reticulum, regulates the membrane potential. In cochlea cells, its number and kinetic properties partly determine the characteristic frequency of each hair cell and thereby helps to establish a tonotopic map. Kinetics of KCNMA1 channels are determined by alternative splicing, phosphorylation status and its combination with modulating beta subunits. Highly sensitive to both iberiotoxin (IbTx) and charybdotoxin (CTX).
- Gene Name:
- KCNMA1
- Uniprot ID:
- Q12791
- Molecular Weight:
- 137558.115 Da
References
- Namba T, Ishii TM, Ikeda M, Hisano T, Itoh T, Hirota K, Adelman JP, Fukuda K: Inhibition of the human intermediate conductance Ca(2+)-activated K(+) channel, hIK1, by volatile anesthetics. Eur J Pharmacol. 2000 Apr 28;395(2):95-101. [10794813 ]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE: The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. [10592235 ]
- General Function:
- Signal transducer activity
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of the calcium.
- Gene Name:
- ATP2C1
- Uniprot ID:
- P98194
- Molecular Weight:
- 100576.42 Da
References
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- This potassium channel may be involved in the regulation of insulin secretion by glucose and/or neurotransmitters acting through G-protein-coupled receptors. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium.
- Gene Name:
- KCNJ6
- Uniprot ID:
- P48051
- Molecular Weight:
- 48450.96 Da
References
- Milovic S, Steinecker-Frohnwieser B, Schreibmayer W, Weigl LG: The sensitivity of G protein-activated K+ channels toward halothane is essentially determined by the C terminus. J Biol Chem. 2004 Aug 13;279(33):34240-9. Epub 2004 Jun 2. [15175324 ]
- Hara K, Yamakura T, Sata T, Harris RA: The effects of anesthetics and ethanol on alpha2 adrenoceptor subtypes expressed with G protein-coupled inwardly rectifying potassium channels in Xenopus oocytes. Anesth Analg. 2005 Nov;101(5):1381-8. [16243998 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
References
- General Function:
- Transmitter-gated ion channel activity
- Specific Function:
- The glycine receptor is a neurotransmitter-gated ion channel. Binding of glycine to its receptor increases the chloride conductance and thus produces hyperpolarization (inhibition of neuronal firing).
- Gene Name:
- GLRA1
- Uniprot ID:
- P23415
- Molecular Weight:
- 52623.35 Da
References
- General Function:
- Protein phosphatase binding
- Specific Function:
- Forms a voltage-independent potassium channel that is activated by intracellular calcium (PubMed:26148990). Activation is followed by membrane hyperpolarization which promotes calcium influx. Required for maximal calcium influx and proliferation during the reactivation of naive T-cells. The channel is blocked by clotrimazole and charybdotoxin but is insensitive to apamin (PubMed:17157250, PubMed:18796614).
- Gene Name:
- KCNN4
- Uniprot ID:
- O15554
- Molecular Weight:
- 47695.12 Da
References
- General Function:
- Nadh dehydrogenase (ubiquinone) activity
- Specific Function:
- Core subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase (Complex I) that is believed to belong to the minimal assembly required for catalysis. Complex I functions in the transfer of electrons from NADH to the respiratory chain. The immediate electron acceptor for the enzyme is believed to be ubiquinone (By similarity).
- Gene Name:
- MT-ND1
- Uniprot ID:
- P03886
- Molecular Weight:
- 35660.055 Da
References
- General Function:
- S100 protein binding
- Specific Function:
- pH-dependent, voltage-insensitive, background potassium channel protein. Rectification direction results from potassium ion concentration on either side of the membrane. Acts as an outward rectifier when external potassium concentration is low. When external potassium concentration is high, current is inward.
- Gene Name:
- KCNK3
- Uniprot ID:
- O14649
- Molecular Weight:
- 43517.665 Da
References
- Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA: Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010 Jun 2;30(22):7691-704. doi: 10.1523/JNEUROSCI.1655-10.2010. [20519544 ]
- Pandit JJ, Buckler KJ: Halothane and sevoflurane exert different degrees of inhibition on carotid body glomus cell intracellular Ca2+ response to hypoxia. Adv Exp Med Biol. 2010;669:201-4. doi: 10.1007/978-1-4419-5692-7_40. [20217349 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- pH-dependent, voltage-insensitive, background potassium channel protein.
- Gene Name:
- KCNK9
- Uniprot ID:
- Q9NPC2
- Molecular Weight:
- 42263.485 Da
References
- Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA: Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010 Jun 2;30(22):7691-704. doi: 10.1523/JNEUROSCI.1655-10.2010. [20519544 ]
- Pandit JJ, Buckler KJ: Halothane and sevoflurane exert different degrees of inhibition on carotid body glomus cell intracellular Ca2+ response to hypoxia. Adv Exp Med Biol. 2010;669:201-4. doi: 10.1007/978-1-4419-5692-7_40. [20217349 ]
- General Function:
- Photoreceptor activity
- Specific Function:
- Photoreceptor required for image-forming vision at low light intensity. Required for photoreceptor cell viability after birth. Light-induced isomerization of 11-cis to all-trans retinal triggers a conformational change leading to G-protein activation and release of all-trans retinal.
- Gene Name:
- RHO
- Uniprot ID:
- P08100
- Molecular Weight:
- 38892.335 Da
References
- Ishizawa Y, Sharp R, Liebman PA, Eckenhoff RG: Halothane binding to a G protein coupled receptor in retinal membranes by photoaffinity labeling. Biochemistry. 2000 Jul 25;39(29):8497-502. [10913255 ]
- Keller C, Grimm C, Wenzel A, Hafezi F, Reme C: Protective effect of halothane anesthesia on retinal light damage: inhibition of metabolic rhodopsin regeneration. Invest Ophthalmol Vis Sci. 2001 Feb;42(2):476-80. [11157886 ]
- General Function:
- Not Available
- Specific Function:
- Keratin-binding protein required for epithelial cell polarization. Involved in apical junction complex (AJC) assembly via its interaction with PARD3. Required for ciliogenesis.
- Gene Name:
- FBF1
- Uniprot ID:
- Q8TES7
- Molecular Weight:
- 125445.19 Da
References
- Bertaccini EJ, Trudell JR, Franks NP: The common chemical motifs within anesthetic binding sites. Anesth Analg. 2007 Feb;104(2):318-24. [17242087 ]
- General Function:
- Pdz domain binding
- Specific Function:
- Ionotropic glutamate receptor. L-glutamate acts as an excitatory neurotransmitter at many synapses in the central nervous system. Binding of the excitatory neurotransmitter L-glutamate induces a conformation change, leading to the opening of the cation channel, and thereby converts the chemical signal to an electrical impulse. The receptor then desensitizes rapidly and enters a transient inactive state, characterized by the presence of bound agonist. In the presence of CACNG4 or CACNG7 or CACNG8, shows resensitization which is characterized by a delayed accumulation of current flux upon continued application of glutamate.
- Gene Name:
- GRIA1
- Uniprot ID:
- P42261
- Molecular Weight:
- 101505.245 Da
References
- Plested AJ, Wildman SS, Lieb WR, Franks NP: Determinants of the sensitivity of AMPA receptors to xenon. Anesthesiology. 2004 Feb;100(2):347-58. [14739810 ]
- General Function:
- Zinc ion binding
- Specific Function:
- NMDA receptor subtype of glutamate-gated ion channels possesses high calcium permeability and voltage-dependent sensitivity to magnesium. Activation requires binding of agonist to both types of subunits.
- Gene Name:
- GRIN2A
- Uniprot ID:
- Q12879
- Molecular Weight:
- 165281.215 Da
References
- Perouansky M, Kirson ED, Yaari Y: Halothane blocks synaptic excitation of inhibitory interneurons. Anesthesiology. 1996 Dec;85(6):1431-8; discussion 29A. [8968191 ]
- General Function:
- Protein phosphatase 2a binding
- Specific Function:
- NMDA receptor subtype of glutamate-gated ion channels with reduced single-channel conductance, low calcium permeability and low voltage-dependent sensitivity to magnesium. Mediated by glycine. May play a role in the development of dendritic spines. May play a role in PPP2CB-NMDAR mediated signaling mechanism (By similarity).
- Gene Name:
- GRIN3A
- Uniprot ID:
- Q8TCU5
- Molecular Weight:
- 125464.07 Da
References
- Perouansky M, Kirson ED, Yaari Y: Halothane blocks synaptic excitation of inhibitory interneurons. Anesthesiology. 1996 Dec;85(6):1431-8; discussion 29A. [8968191 ]
- General Function:
- Nmda glutamate receptor activity
- Specific Function:
- NMDA receptor subtype of glutamate-gated ion channels with reduced single-channel conductance, low calcium permeability and low voltage-dependent sensitivity to magnesium. Mediated by glycine.
- Gene Name:
- GRIN3B
- Uniprot ID:
- O60391
- Molecular Weight:
- 112990.98 Da
References
- Perouansky M, Kirson ED, Yaari Y: Halothane blocks synaptic excitation of inhibitory interneurons. Anesthesiology. 1996 Dec;85(6):1431-8; discussion 29A. [8968191 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Potassium channel activated by both membrane depolarization or increase in cytosolic Ca(2+) that mediates export of K(+). It is also activated by the concentration of cytosolic Mg(2+). Its activation dampens the excitatory events that elevate the cytosolic Ca(2+) concentration and/or depolarize the cell membrane. It therefore contributes to repolarization of the membrane potential. Plays a key role in controlling excitability in a number of systems, such as regulation of the contraction of smooth muscle, the tuning of hair cells in the cochlea, regulation of transmitter release, and innate immunity. In smooth muscles, its activation by high level of Ca(2+), caused by ryanodine receptors in the sarcoplasmic reticulum, regulates the membrane potential. In cochlea cells, its number and kinetic properties partly determine the characteristic frequency of each hair cell and thereby helps to establish a tonotopic map. Kinetics of KCNMA1 channels are determined by alternative splicing, phosphorylation status and its combination with modulating beta subunits. Highly sensitive to both iberiotoxin (IbTx) and charybdotoxin (CTX).
- Gene Name:
- KCNMA1
- Uniprot ID:
- Q12791
- Molecular Weight:
- 137558.115 Da
References
- Namba T, Ishii TM, Ikeda M, Hisano T, Itoh T, Hirota K, Adelman JP, Fukuda K: Inhibition of the human intermediate conductance Ca(2+)-activated K(+) channel, hIK1, by volatile anesthetics. Eur J Pharmacol. 2000 Apr 28;395(2):95-101. [10794813 ]
22. GABA-A receptor (anion channel) (Protein Group)
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Included Proteins:
- P14867 , P47869 , P34903 , P48169 , P31644 , Q16445 , P18505 , P47870 , P28472 , O14764 , P78334 , Q8N1C3 , P18507 , Q99928 , O00591 , Q9UN88