| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2010-05-05 19:41:24 UTC |
|---|
| Update Date | 2014-12-24 20:26:28 UTC |
|---|
| Accession Number | T3D3736 |
|---|
| Identification |
|---|
| Common Name | Cyclopiazonic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Cyclopiazonic acid (CPA) is a myxotoxin originally isolated from Penicillium cyclopium and subsequently from Penicillium griseofulvum, Penicillium camembertii, Aspergillus flavus and Aspergillus versicolor. It is often found co-occurring with aflatoxins. CPA is a natural contaminant of corn, peanuts, and sunflowers, as well as various types of cheese, sausages, and salamis. It may also occur in eggs, milk and meats from animal that have consumed CPA-contaminated feed and is toxic in high concentrations. (7, 2)
|
|---|
| Compound Type | - Amide
- Amine
- Food Toxin
- Fungal Toxin
- Mycotoxin
- Natural Compound
- Organic Compound
|
|---|
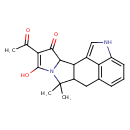
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | .alpha.-Cyclopiazonic acid | | 10-Acetyl-11-hydroxy-7,7-dimethyl-2,6,6a,7,11a,11b-hexahydro-9H-pyrrolo[1',2':2,3]isoindolo[4,5,6-cd]indol-9-one | | alpha-Cyclopiazonate | | alpha-Cyclopiazonic acid | | CPA | | Cyclopiazonate |
|
|---|
| Chemical Formula | C20H20N2O3 |
|---|
| Average Molecular Mass | 336.384 g/mol |
|---|
| Monoisotopic Mass | 336.147 g/mol |
|---|
| CAS Registry Number | 18172-33-3 |
|---|
| IUPAC Name | 5-acetyl-6-hydroxy-8,8-dimethyl-7,16-diazapentacyclo[9.6.1.0²,⁹.0³,⁷.0¹⁵,¹⁸]octadeca-1(17),5,11(18),12,14-pentaen-4-one |
|---|
| Traditional Name | 5-acetyl-6-hydroxy-8,8-dimethyl-7,16-diazapentacyclo[9.6.1.0²,⁹.0³,⁷.0¹⁵,¹⁸]octadeca-1(17),5,11(18),12,14-pentaen-4-one |
|---|
| SMILES | CC(=O)C1=C(O)N2C(C3C(CC4=C5C(NC=C35)=CC=C4)C2(C)C)C1=O |
|---|
| InChI Identifier | InChI=1S/C20H20N2O3/c1-9(23)14-18(24)17-16-11-8-21-13-6-4-5-10(15(11)13)7-12(16)20(2,3)22(17)19(14)25/h4-6,8,12,16-17,21,25H,7H2,1-3H3 |
|---|
| InChI Key | InChIKey=RLOAZVAJNNPPDI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-alkylindoles. 3-alkylindoles are compounds containing an indole moiety that carries an alkyl chain at the 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indoles |
|---|
| Direct Parent | 3-alkylindoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-alkylindole
- Isoindole or derivatives
- Isoindoline
- Pyrrolizine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Vinylogous acid
- Pyrroline
- Pyrrolidine
- Pyrrole
- Ketone
- Ketene acetal or derivatives
- Azacycle
- Alkanolamine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0049000000-beed7ad8d422dddd235e | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fri-0139000000-74b4d3a12818ba276b0f | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0930000000-0c3b3885c6f053326fc9 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-0069000000-f82fe95bc7b5e0693f15 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1091000000-c68a07a4a2e1691b83c8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0600-9180000000-429a3089cb5b2f2add85 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral, dermal, inhalation, and parenteral (contaminated drugs). (6) |
|---|
| Mechanism of Toxicity | Cyclopiazonic acid is potent and specific inhibitor of the endoplasmic and sarcoplasmic reticulum Ca+-dependent ATPases, which are essential for calcium reuptake in the muscle contraction-relaxation cycle. CPA blocks the calcium access channel and rigidifies a subset of transmembrane helices in a nonnative configuration that is incompatible with calcium binding. Inhibition of Ca2+-ATPases results in cell death through the activation of stress-response and apoptotic pathways within the endoplasmic reticulum and mitochondria. CPA can also induce both secretion and mRNA levels of proinflammatory cytokines, likely leading to macrophage activation and immunotoxic effects. In addition, it has been shown to be mutagenic and genotoxic. Mycotoxins are often able to enter the liver and kidney by human organic anion transporters (hOATs) and human organic cation transporters (hOCTs). They can also inhibit uptake of anions and cations by these transporters, interefering with the secretion of endogenous metabolites, drugs, and xenobiotics including themselves. This results in increased cellular accumulation of toxic compounds causing nephro- and hepatotoxicity. (1, 2, 4, 5) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | LD50: 13 mg/kg (Intraperitoneal, Mouse) (3)
LD50: 2.3 mg/kg (Intraperitoneal, Rat) (4) |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Cyclopiazonic acid (CPA) is a myxotoxin originally isolated from Penicillium cyclopium and subsequently from Penicillium griseofulvum, Penicillium camembertii, Aspergillus flavus and Aspergillus versicolor. It is often found co-occurring with aflatoxins. CPA is a natural contaminant of corn, peanuts, and sunflowers, as well as various types of cheese, sausages, and salamis. It may also occur in eggs, milk and meats from animal that have consumed CPA-contaminated feed. (7, 2) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Cyclopiazonic acid affects muscle function and may also be immunotoxic, genotoxic, and mutagenic. (2, 3, 4) |
|---|
| Symptoms | Animals studies have shown cyclopiazonic acid to cause symptoms such as tremors, convulsions, dyspnoea, hypokinesia, hypothermia, sedation, tachycardia, and tachypnoea. (3) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 2908 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 2805 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Cyclopiazonic_acid |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3736.pdf |
|---|
| General References | - Moncoq K, Trieber CA, Young HS: The molecular basis for cyclopiazonic acid inhibition of the sarcoplasmic reticulum calcium pump. J Biol Chem. 2007 Mar 30;282(13):9748-57. Epub 2007 Jan 26. [17259168 ]
- Marin ML, Wong SS, Pestka JJ: Increased IL-1, IL-6 and TNF alpha secretion and mRNA levels in WEHI-3 cells exposed to cyclopiazonic acid. Toxicology. 1996 Nov 15;114(1):67-79. [8931762 ]
- Nishie K, Cole RJ, Dorner JW: Toxicity and neuropharmacology of cyclopiazonic acid. Food Chem Toxicol. 1985 Sep;23(9):831-9. [4043883 ]
- Sorenson WG, Tucker JD, Simpson JP: Mutagenicity of tetramic mycotoxin cyclopiazonic acid. Appl Environ Microbiol. 1984 Jun;47(6):1355-7. [6430233 ]
- Tachampa K, Takeda M, Khamdang S, Noshiro-Kofuji R, Tsuda M, Jariyawat S, Fukutomi T, Sophasan S, Anzai N, Endou H: Interactions of organic anion transporters and organic cation transporters with mycotoxins. J Pharmacol Sci. 2008 Mar;106(3):435-43. Epub 2008 Mar 5. [18319568 ]
- Peraica M, Domijan AM: Contamination of food with mycotoxins and human health. Arh Hig Rada Toksikol. 2001 Mar;52(1):23-35. [11370295 ]
- Wikipedia. Cyclopiazonic acid. Last Updated 13 February 2010. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|