| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2013-04-25 07:56:53 UTC |
|---|
| Update Date | 2014-12-24 20:26:34 UTC |
|---|
| Accession Number | T3D3902 |
|---|
| Identification |
|---|
| Common Name | Primisulfuron-methyl |
|---|
| Class | Small Molecule |
|---|
| Description | Primisulfuron-methyl is a urea herbicide that is used on crop and non- crop areas for the control of grass weeds and many kinds of broad-leaved weeds. The compound is a selective postemergence systemic herbicide that is rapidly absorbed by plants and transported throughout the plant roots and foliage. The herbicide works by blocking cell growth in the active growing regions of the plant. The herbicide also acts to inhibit photosynthesis |
|---|
| Compound Type | - Amide
- Amine
- Ester
- Ether
- Herbicide
- Organic Compound
- Organofluoride
- Pesticide
- Synthetic Compound
|
|---|
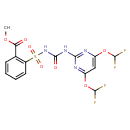
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Beacon | | Methyl 2-({[4,6-bis(difluoromethoxy)-2-pyrimidinyl]carbamoyl}sulfamoyl)benzoate | | Methyl 2-[({[4,6-bis(difluoromethoxy)pyrimidin-2-yl]carbamoyl}amino)sulfonyl]benzoate | | Methyl 2-[[[[[4,6-bis(difluoromethoxy)-2-pyrimidinyl]amino]carbonyl]amino]sulfonyl]benzoate | | Methyl 2-{[({[4,6-bis(difluoromethoxy)-2-pyrimidinyl]amino}carbonyl)amino]sulfonyl}benzoate | | Rifle | | Tell |
|
|---|
| Chemical Formula | C15H12F4N4O7S |
|---|
| Average Molecular Mass | 468.337 g/mol |
|---|
| Monoisotopic Mass | 468.036 g/mol |
|---|
| CAS Registry Number | 86209-51-0 |
|---|
| IUPAC Name | methyl 2-[({[4,6-bis(difluoromethoxy)pyrimidin-2-yl]carbamoyl}amino)sulfonyl]benzoate |
|---|
| Traditional Name | primisulfuron-methyl |
|---|
| SMILES | COC(=O)C1=C(C=CC=C1)S(=O)(=O)NC(=O)NC1=NC(OC(F)F)=CC(OC(F)F)=N1 |
|---|
| InChI Identifier | InChI=1S/C15H12F4N4O7S/c1-28-11(24)7-4-2-3-5-8(7)31(26,27)23-15(25)22-14-20-9(29-12(16)17)6-10(21-14)30-13(18)19/h2-6,12-13H,1H3,(H2,20,21,22,23,25) |
|---|
| InChI Key | InChIKey=ZTYVMAQSHCZXLF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidinyl-2-sulfonylureas. These are aromatic heterocyclic compounds containing a pyrimidine ring which is substituted with a sulfonylurea at the ring 2-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Sulfonylureas |
|---|
| Direct Parent | Pyrimidinyl-2-sulfonylureas |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidinyl-2-sulfonylurea
- Benzenesulfonamide
- Benzoate ester
- Benzoic acid or derivatives
- Benzenesulfonyl group
- Benzoyl
- Monocyclic benzene moiety
- Pyrimidine
- Benzenoid
- Methyl ester
- Aminosulfonyl compound
- Sulfonyl
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Heteroaromatic compound
- Carboxylic acid ester
- Carbonic acid derivative
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Azacycle
- Alkyl fluoride
- Organohalogen compound
- Organofluoride
- Organooxygen compound
- Carbonyl group
- Organosulfur compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Alkyl halide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fb9-2690600000-cebb4f57ee32c502e5e0 | 2021-09-24 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0150900000-cade24e6240f98177a83 | 2016-06-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-5490100000-906faee99c8752bc36c7 | 2016-06-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dr-2920000000-a0dbbe1c509b7d4df76b | 2016-06-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016r-0090700000-966355b6145a0d07445d | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dl-6490100000-cf5a664166f8a0bf6ca5 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9430000000-fd31165989d4f323669d | 2016-08-04 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 101525 |
|---|
| ChEMBL ID | CHEMBL1875740 |
|---|
| ChemSpider ID | 91738 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3902.pdf |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|