| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-26 22:04:07 UTC |
|---|
| Update Date | 2014-12-24 20:22:40 UTC |
|---|
| Accession Number | T3D0718 |
|---|
| Identification |
|---|
| Common Name | Potassium zinc chromate hydroxide |
|---|
| Class | Small Molecule |
|---|
| Description | Potassium zinc chromate hydroxide is a chemical compound of zinc, potassium and hexavalent chromium. Zinc is a metallic element with the atomic number 30. It is found in nature most often as the mineral sphalerite. Though excess zinc in harmful, in smaller amounts it is an essential element for life, as it is a cofactor for over 300 enzymes and is found in just as many transcription factors. Hexavalent chromium refers to chemical compounds that contain the element chromium in the +6 oxidation state. Chromium(VI) is more toxic than other oxidation states of the chromium atom because of its greater ability to enter cells and higher redox potential. (7, 8, 9) |
|---|
| Compound Type | - Chromium Compound
- Industrial/Workplace Toxin
- Inorganic Compound
- Pollutant
- Synthetic Compound
- Zinc Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Chromic acid, potassium zinc salt (2:2:1) | | Potassium hydroxyoctaoxodizincatedichromate(1-) | | Potassium zinc chromate | | Potassium zinc chromate hydroxide | | Potassium zinc chromate hydroxide (KZn2(CrO4)2(OH)) | | Zinc chromate | | Zinc potassium chromate hydroxide | | Zinc potassium chromic acid | | Zinc yellow |
|
|---|
| Chemical Formula | Cr2HKO9Zn2 |
|---|
| Average Molecular Mass | 418.911 g/mol |
|---|
| Monoisotopic Mass | 415.665 g/mol |
|---|
| CAS Registry Number | 11103-86-9 |
|---|
| IUPAC Name | dizinc(2+) ion potassium bis(dioxochromiumbis(olate)) hydroxide |
|---|
| Traditional Name | dizinc(2+) ion potassium bis(chromate(2-)) hydroxide |
|---|
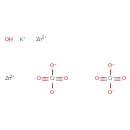
| SMILES | [OH-].[K+].[Zn++].[Zn++].[O-][Cr]([O-])(=O)=O.[O-][Cr]([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/2Cr.K.H2O.8O.2Zn/h;;;1H2;;;;;;;;;;/q;;+1;;;;;;4*-1;2*+2/p-1 |
|---|
| InChI Key | InChIKey=GQKCRUJOPUHISR-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as miscellaneous chromates. These are inorganic compounds in which the largest metallic oxoanion is chromate, to which either no atom or a non metal atom is bonded. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Miscellaneous mixed metal/non-metals |
|---|
| Sub Class | Miscellaneous metallic oxoanionic compounds |
|---|
| Direct Parent | Miscellaneous chromates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chromate
- Alkali metal chromate
- Inorganic hydride
- Inorganic oxide
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Endoplasmic reticulum
- Extracellular
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Base excision repair | Not Available | map03410 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Green-yellow solid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (9) ; oral (9) ; dermal (9) |
|---|
| Mechanism of Toxicity | Anaemia results from the excessive absorption of zinc suppressing copper and iron absorption, most likely through competitive binding of intestinal mucosal cells. Unbalanced levels of copper and zinc binding to Cu,Zn-superoxide dismutase has been linked to amyotrophic lateral sclerosis (ALS). Stomach acid dissolves metallic zinc to give corrosive zinc chloride, which can cause damage to the stomach lining. Metal fume fever is thought to be an immune response to inhaled zinc. Hexavalent chromium's carcinogenic effects are caused by its metabolites, pentavalent and trivalent chromium. The DNA damage may be caused by hydroxyl radicals produced during reoxidation of pentavalent chromium by hydrogen peroxide molecules present in the cell. Trivalent chromium may also form complexes with peptides, proteins, and DNA, resulting in DNA-protein crosslinks, DNA strand breaks, DNA-DNA interstrand crosslinks, chromium-DNA adducts, chromosomal aberrations and alterations in cellular signaling pathways. It has been shown to induce carcinogenesis by overstimulating cellular regulatory pathways and increasing peroxide levels by activating certain mitogen-activated protein kinases. It can also cause transcriptional repression by cross-linking histone deacetylase 1-DNA methyltransferase 1 complexes to CYP1A1 promoter chromatin, inhibiting histone modification. Chromium may increase its own toxicity by modifying metal regulatory transcription factor 1, causing the inhibition of zinc-induced metallothionein transcription. (1, 7, 2, 3, 4, 8, 9, 5) |
|---|
| Metabolism | Zinc can enter the body through the lungs, skin, and gastrointestinal tract. Intestinal absorption of zinc is controlled by zinc carrier protein CRIP. Zinc also binds to metallothioneins, which help prevent absorption of excess zinc. Zinc is widely distributed and found in all tissues and tissues fluids, concentrating in the liver, gastrointestinal tract, kidney, skin, lung, brain, heart, and pancreas. In the bloodstream zinc is found bound to carbonic anhydrase in erythrocytes, as well as bound to albumin, _2-macroglobulin, and amino acids in the the plasma. Albumin and amino acid bound zinc can diffuse across tissue membranes. Zinc is excreted in the urine and faeces. Chromium is absorbed from oral, inhalation, or dermal exposure and distributes to nearly all tissues, with the highest concentrations found in kidney and liver. Bone is also a major storage site and may contribute to long-term retention. Hexavalent chromium's similarity to sulfate and chromate allow it to be transported into cells via sulfate transport mechanisms. Inside the cell, hexavalent chromium is reduced first to pentavalent chromium, then to trivalent chromium by many substances including ascorbate, glutathione, and nicotinamide adenine dinucleotide. Chromium is almost entirely excreted with the urine. (1, 7, 9) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | 1 to 3 grams for an adult human (hexavalent chromium). (6) |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (12) |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Intermediate Oral: 0.3 mg/kg/day (Zinc) (11)
Chronic Oral: 0.3 mg/kg/day (Zinc) (11)
Intermediate Oral: 0.005 mg/kg/day (Hexavalent Chromium) (11)
Chronic Oral: 0.001 mg/kg/day (Hexavalent Chromium) (11) |
|---|
| Health Effects | Chronic exposure to zinc causes anemia, atazia, lethargy, and decreases the level of good cholesterol in the body. It is also believed to cause pancreatic and reproductive damage. Hexavalent chromium is a known carcinogen. Chronic inhalation especially has been linked to lung cancer. Hexavalent chromium has also been know to cause reproductive and developmental defects. (1, 9) |
|---|
| Symptoms | Ingestion of large doses of zinc causes stomach cramps, nausea, and vomiting. Acute inhalation of large amounts of zinc causes metal fume fever, which is characterized by chills, fever, headache, weakness, dryness of the nose and throat, chest pain, and coughing. Dermal contact with zinc results in skin irritation. Breathing hexavalent chromium can cause irritation to the lining of the nose, nose ulcers, runny nose, and breathing problems, such as asthma, cough, shortness of breath, or wheezing. Ingestion of hexavalent chromium causes irritation and ulcers in the stomach and small intestine, as well as anemia. Skin contact can cause skin ulcers. (7, 9) |
|---|
| Treatment | Zinc poisoning is treated symptomatically, often by administering fluids such as water or milk, or with gastric lavage. There is no know antidote for chromium poisoning. Exposure is usually handled with symptomatic treatment. (7, 9) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 25466 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 23351080 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Zinc potassium chromate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0718.pdf |
|---|
| General References | - Salnikow K, Zhitkovich A: Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008 Jan;21(1):28-44. Epub 2007 Oct 30. [17970581 ]
- Kim G, Yurkow EJ: Chromium induces a persistent activation of mitogen-activated protein kinases by a redox-sensitive mechanism in H4 rat hepatoma cells. Cancer Res. 1996 May 1;56(9):2045-51. [8616849 ]
- Schnekenburger M, Talaska G, Puga A: Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007 Oct;27(20):7089-101. Epub 2007 Aug 6. [17682057 ]
- Kimura T: [Molecular mechanism involved in chromium(VI) toxicity]. Yakugaku Zasshi. 2007 Dec;127(12):1957-65. [18057785 ]
- Vonk WI, Klomp LW: Role of transition metals in the pathogenesis of amyotrophic lateral sclerosis. Biochem Soc Trans. 2008 Dec;36(Pt 6):1322-8. doi: 10.1042/BST0361322. [19021549 ]
- Barceloux DG: Chromium. J Toxicol Clin Toxicol. 1999;37(2):173-94. [10382554 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for chromium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Zinc. Last Updated 24 March 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2005). Toxicological profile for zinc. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Metallothionein. Last Updated 20 December 2008. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|