Acrinathrin (T3D1029)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-06-18 17:03:35 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:23:04 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D1029 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Acrinathrin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | A pyrethroid is a synthetic chemical compound similar to the natural chemical pyrethrins produced by the flowers of pyrethrums (Chrysanthemum cinerariaefolium and C. coccineum). Pyrethroids are common in commercial products such as household insecticides and insect repellents. In the concentrations used in such products, they are generally harmless to human beings but can harm sensitive individuals. They are usually broken apart by sunlight and the atmosphere in one or two days, and do not significantly affect groundwater quality except for being toxic to fish. (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
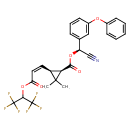
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C26H21F6NO5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 541.439 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 541.132 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 101007-06-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (S)-cyano(3-phenoxyphenyl)methyl (1R,3S)-3-[(1Z)-3-[(1,1,1,3,3,3-hexafluoropropan-2-yl)oxy]-3-oxoprop-1-en-1-yl]-2,2-dimethylcyclopropane-1-carboxylate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | acrinathrin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | N#C[C@@H](OC(=O)[C@H]1C(C)(C)[C@H]1\C=C/C(=O)OC(C(F)(F)F)C(F)(F)F)C1=CC=CC(OC2=CC=CC=C2)=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C26H21F6NO5/c1-24(2)18(11-12-20(34)38-23(25(27,28)29)26(30,31)32)21(24)22(35)37-19(14-33)15-7-6-10-17(13-15)36-16-8-4-3-5-9-16/h3-13,18-19,21,23H,1-2H3/b12-11-/t18-,19+,21-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=YLFSVIMMRPNPFK-WEQBUNFVSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as pyrethroids. These are organic compounds similar to the pyrethrins. Some pyrethroids containing a chrysanthemic acid esterified with a cyclopentenone (pyrethrins), or with a phenoxybenzyl group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty acid esters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrethroids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Both type I and type II pyrethroids exert their effect by prolonging the open phase of the sodium channel gates when a nerve cell is excited. They appear to bind to the membrane lipid phase in the immediate vicinity of the sodium channel, thus modifying the channel kinetics. This blocks the closing of the sodium gates in the nerves, and thus prolongs the return of the membrane potential to its resting state. The repetitive (sensory, motor) neuronal discharge and a prolonged negative afterpotential produces effects quite similar to those produced by DDT, leading to hyperactivity of the nervous system which can result in paralysis and/or death. Other mechanisms of action of pyrethroids include antagonism of gamma-aminobutyric acid (GABA)-mediated inhibition, modulation of nicotinic cholinergic transmission, enhancement of noradrenaline release, and actions on calcium ions. They also inhibit calium channels and Ca2+, Mg2+-ATPase. (2, 3, 6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Following ingestion, pyrethriods are hydrolysed by various digestive enzymes in the gastro-intestinal tract. However, a small portion of the insecticidally active compounds or its derivatives are absorbed, as shown by their toxicity and their effect on the liver. Pyrethriods may also be absorbed following inhalation or dermal contact. They are rapidly distributed to most tissues, particularly to those with a high lipid content, and are concentrated in central and peripheral nervous tissues. Pyrethriods or their metabolites are not known to be stored in the body or to be excreted in the milk, but no study of the matter has employed modern methods. The major metabolic pathways for pyrethriods are hydrolysis of the central ester bond, oxidative attacks at several sites, and conjugation reactions, to produce a complex array of primary and secondary water-soluble metabolites that undergo urinary excretion. Metabolism is believed to involve nonspecific microsomal carboxyesterases and microsomal mixed function oxidases, which are located in nearly all tissue types, with particularly high activities in the liver. Metabolites are excreted in the urine and faeces. (6, 7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Pyrethroids are used as insecticides. (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Pyrethroid effects typically include rapid onset of aggressive behavior and increased sensitivity to external stimuli, followed by fine tremor, prostration with coarse whole body tremor, elevated body temperature, coma, and death. Paresthesia, severe corneal damage, hypotension and tachycardia, associated with anaphylaxis, can also occur following pyrethriod poisoning. (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Spilling on the head, face and eyes can result in pain, lacrimation, photophobia, congestion, and edema of the conjunctiva and eyelids. Ingestion cases epigastric pain, nausea, vomiting, headache, dizziness, anorexia, fatigue, tightness in chest, blurred vision, paresthesia, palpitations, coarse muscular fasciculations, and disturbances of conciousness. In servere poisonings, convulsive attacks with opisthotonos and loss of conciousness have occurred. (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Following oral exposure, the treatment is symptomatic and supportive and includes monitoring for the development of hypersensitivity reactions with respiratory distress. Provide adequate airway management when needed. Gastric decontamination is usually not required unless the pyrethrin product is combined with a hydrocarbon. Following inhalation exposure, move patient to fresh air. monitor for respiratory distress. If cough or difficulty breathing develops, evaluate for respiratory tract irritation, bronchitis, or pneumonitis. Administer oxygen and assist ventilation as required. Treat bronchospasm with inhaled beta2 agonist and oral or parenteral corticosteroids. In case of eye exposure, irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes. If irritation, pain, swelling, lacrimation, or photophobia persist, the patient should be seen in a health care facility. If the contamination occurs through dermal exposure, Remove contaminated clothing and wash exposed area thoroughly with soap and water. A physician may need to examine the area if irritation or pain persists. Vitamin E topical application is highly effective in relieving parenthesis. (8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 6436606 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL2287507 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 4941233 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C18408 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 39091 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Acrinathrin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D1029.pdf | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- Mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient.
- Gene Name:
- SCN1A
- Uniprot ID:
- P35498
- Molecular Weight:
- 228969.49 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- Tetrodotoxin-resistant channel that mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which sodium ions may pass in accordance with their electrochemical gradient. Plays a role in neuropathic pain mechanisms.
- Gene Name:
- SCN10A
- Uniprot ID:
- Q9Y5Y9
- Molecular Weight:
- 220623.605 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- This protein mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which sodium ions may pass in accordance with their electrochemical gradient. It is a tetrodotoxin-resistant sodium channel isoform. Also involved, with the contribution of the receptor tyrosine kinase NTRK2, in rapid BDNF-evoked neuronal depolarization.
- Gene Name:
- SCN11A
- Uniprot ID:
- Q9UI33
- Molecular Weight:
- 204919.66 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- Mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient.
- Gene Name:
- SCN2A
- Uniprot ID:
- Q99250
- Molecular Weight:
- 227972.64 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- Mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient.
- Gene Name:
- SCN3A
- Uniprot ID:
- Q9NY46
- Molecular Weight:
- 226291.905 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- This protein mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient. This sodium channel may be present in both denervated and innervated skeletal muscle.
- Gene Name:
- SCN4A
- Uniprot ID:
- P35499
- Molecular Weight:
- 208059.175 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity involved in sa node cell action potential
- Specific Function:
- This protein mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient. It is a tetrodotoxin-resistant Na(+) channel isoform. This channel is responsible for the initial upstroke of the action potential. Channel inactivation is regulated by intracellular calcium levels.
- Gene Name:
- SCN5A
- Uniprot ID:
- Q14524
- Molecular Weight:
- 226937.475 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- Mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient.
- Gene Name:
- SCN7A
- Uniprot ID:
- Q01118
- Molecular Weight:
- 193491.605 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- Mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient. In macrophages and melanoma cells, isoform 5 may participate in the control of podosome and invadopodia formation.
- Gene Name:
- SCN8A
- Uniprot ID:
- Q9UQD0
- Molecular Weight:
- 225278.005 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity
- Specific Function:
- Mediates the voltage-dependent sodium ion permeability of excitable membranes. Assuming opened or closed conformations in response to the voltage difference across the membrane, the protein forms a sodium-selective channel through which Na(+) ions may pass in accordance with their electrochemical gradient. It is a tetrodotoxin-sensitive Na(+) channel isoform. Plays a role in pain mechanisms, especially in the development of inflammatory pain (By similarity).
- Gene Name:
- SCN9A
- Uniprot ID:
- Q15858
- Molecular Weight:
- 226370.175 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity involved in purkinje myocyte action potential
- Specific Function:
- Crucial in the assembly, expression, and functional modulation of the heterotrimeric complex of the sodium channel. The subunit beta-1 can modulate multiple alpha subunit isoforms from brain, skeletal muscle, and heart. Its association with neurofascin may target the sodium channels to the nodes of Ranvier of developing axons and retain these channels at the nodes in mature myelinated axons.Isoform 2: Cell adhesion molecule that plays a critical role in neuronal migration and pathfinding during brain development. Stimulates neurite outgrowth.
- Gene Name:
- SCN1B
- Uniprot ID:
- Q07699
- Molecular Weight:
- 24706.955 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity involved in cardiac muscle cell action potential
- Specific Function:
- Crucial in the assembly, expression, and functional modulation of the heterotrimeric complex of the sodium channel. The subunit beta-2 causes an increase in the plasma membrane surface area and in its folding into microvilli. Interacts with TNR may play a crucial role in clustering and regulation of activity of sodium channels at nodes of Ranvier (By similarity).
- Gene Name:
- SCN2B
- Uniprot ID:
- O60939
- Molecular Weight:
- 24325.69 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity involved in cardiac muscle cell action potential
- Specific Function:
- Modulates channel gating kinetics. Causes unique persistent sodium currents. Inactivates the sodium channel opening more slowly than the subunit beta-1. Its association with neurofascin may target the sodium channels to the nodes of Ranvier of developing axons and retain these channels at the nodes in mature myelinated axons (By similarity).
- Gene Name:
- SCN3B
- Uniprot ID:
- Q9NY72
- Molecular Weight:
- 24702.08 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated sodium channel activity involved in cardiac muscle cell action potential
- Specific Function:
- Modulates channel gating kinetics. Causes negative shifts in the voltage dependence of activation of certain alpha sodium channels, but does not affect the voltage dependence of inactivation. Modulates the suceptibility of the sodium channel to inhibition by toxic peptides from spider, scorpion, wasp and sea anemone venom.
- Gene Name:
- SCN4B
- Uniprot ID:
- Q8IWT1
- Molecular Weight:
- 24968.755 Da
References
- Everts HB, Sundberg JP, Ong DE: Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005 Aug 15;308(2):309-19. [15950969 ]
- Klaassen CD, Amdur MO, Doull J (eds) (1995). Casarett and Doull's Toxicology. The Basic Science of Poisons. 5th ed. New York, NY: McGraw-Hill.
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for pyrethrins and pyrethroids. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Signal transducer activity
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of the calcium.
- Gene Name:
- ATP2C1
- Uniprot ID:
- P98194
- Molecular Weight:
- 100576.42 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium.
- Gene Name:
- ATP2C2
- Uniprot ID:
- O75185
- Molecular Weight:
- 103186.475 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Protein homodimerization activity
- Specific Function:
- Key regulator of striated muscle performance by acting as the major Ca(2+) ATPase responsible for the reuptake of cytosolic Ca(2+) into the sarcoplasmic reticulum. Catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A1
- Uniprot ID:
- O14983
- Molecular Weight:
- 110251.36 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- S100 protein binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Isoform 2 is involved in the regulation of the contraction/relaxation cycle.
- Gene Name:
- ATP2A2
- Uniprot ID:
- P16615
- Molecular Weight:
- 114755.765 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium. Transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A3
- Uniprot ID:
- Q93084
- Molecular Weight:
- 113976.23 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.