Alprazolam (T3D2777)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:26:48 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:51 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2777 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Alprazolam | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Alprazolam is only found in individuals that have used or taken this drug. It is a triazolobenzodiazepine compound with antianxiety and sedative-hypnotic actions, that is efficacious in the treatment of panic disorders, with or without agoraphobia, and in generalized anxiety disorders. (From AMA Drug Evaluations Annual, 1994, p238) Benzodiazepines bind nonspecifically to benzodiazepine receptors BNZ1, which mediates sleep, and BNZ2, which affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
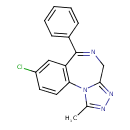
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C17H13ClN4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 308.765 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 308.083 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 28981-97-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 12-chloro-3-methyl-9-phenyl-2,4,5,8-tetraazatricyclo[8.4.0.0²,⁶]tetradeca-1(10),3,5,8,11,13-hexaene | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | neurol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CC1=NN=C2CN=C(C3=CC=CC=C3)C3=C(C=CC(Cl)=C3)N12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C17H13ClN4/c1-11-20-21-16-10-19-17(12-5-3-2-4-6-12)14-9-13(18)7-8-15(14)22(11)16/h2-9H,10H2,1H3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=VREFGVBLTWBCJP-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as 1,2,4-triazolo[4,3-a][1,4]benzodiazepines. These are aromatic compounds containing a 1,4-benzodiazepine fused to and sharing a nitrogen atom with a 1,2,4-triazole ring. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzodiazepines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | 1,4-benzodiazepines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 1,2,4-triazolo[4,3-a][1,4]benzodiazepines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral. Readily absorbed from the gastrointestinal tract. Bioavailability is 80-90%. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Benzodiazepines bind nonspecifically to benzodiazepine receptors BNZ1, which mediates sleep, and BNZ2, which affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Hepatic. Hydroxylated in the liver to α-hydroxyalprazolam, which is also active. This and other metabolites are later excreted in urine as glucuronides. Route of Elimination: Alprazolam and its metabolites are excreted primarily in the urine. Half Life: 6.3-26.9 hours | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 1020 mg/kg (Oral, mouse) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For the management of anxiety disorder or the short-term relief of symptoms of anxiety and for the treatment of panic disorder, with or without agoraphobia. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of overdose include confusion, coma, impaired coordination, sleepiness, and slowed reaction time. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | As in all cases of drug overdosage, respiration, pulse rate, and blood pressure should be monitored. General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. If hypotension occurs, it may be combated by the use of vasopressors. Dialysis is of limited value. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. (9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00404 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB14548 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 2118 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL661 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 2034 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C06817 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 2611 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Alprazolam | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | 08H | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Alprazolam | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Hester, J.B., Jr.; US. Patent 3,681,343; August 1,1972; assigned to The Upjohn Company. Hester, J.B., Jr.; US.Patent 3,781,289; December 25,1973;assigned to The Upjohn Company. Hester, J.B., Jr.; U S . Patent 3,709898; January 9,1973; assigned to The Upjohn Company. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA2
- Uniprot ID:
- P47869
- Molecular Weight:
- 51325.85 Da
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA3
- Uniprot ID:
- P34903
- Molecular Weight:
- 55164.055 Da
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Transporter activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA5
- Uniprot ID:
- P31644
- Molecular Weight:
- 52145.645 Da
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
4. GABA-A receptor (anion channel) (Protein Group)
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Included Proteins:
- P14867 , P47869 , P34903 , P48169 , P31644 , Q16445 , P18505 , P47870 , P28472 , O14764 , P78334 , Q8N1C3 , P18507 , Q99928 , O00591 , Q9UN88
References
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Verster JC, Volkerts ER: Clinical pharmacology, clinical efficacy, and behavioral toxicity of alprazolam: a review of the literature. CNS Drug Rev. 2004 Spring;10(1):45-76. [14978513 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
References
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA4
- Uniprot ID:
- P48169
- Molecular Weight:
- 61622.645 Da
References
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA6
- Uniprot ID:
- Q16445
- Molecular Weight:
- 51023.69 Da
References
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRB1
- Uniprot ID:
- P18505
- Molecular Weight:
- 54234.085 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB2
- Uniprot ID:
- P47870
- Molecular Weight:
- 59149.895 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-gated chloride ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB3
- Uniprot ID:
- P28472
- Molecular Weight:
- 54115.04 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRD
- Uniprot ID:
- O14764
- Molecular Weight:
- 50707.835 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRE
- Uniprot ID:
- P78334
- Molecular Weight:
- 57971.175 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG1
- Uniprot ID:
- Q8N1C3
- Molecular Weight:
- 53594.49 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRG2
- Uniprot ID:
- P18507
- Molecular Weight:
- 54161.78 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG3
- Uniprot ID:
- Q99928
- Molecular Weight:
- 54288.16 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. In the uterus, the function of the receptor appears to be related to tissue contractility. The binding of this pI subunit with other GABA(A) receptor subunits alters the sensitivity of recombinant receptors to modulatory agents such as pregnanolone.
- Gene Name:
- GABRP
- Uniprot ID:
- O00591
- Molecular Weight:
- 50639.735 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-1 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR1
- Uniprot ID:
- P24046
- Molecular Weight:
- 55882.91 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-2 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR2
- Uniprot ID:
- P28476
- Molecular Weight:
- 54150.41 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Platelet activating factor receptor activity
- Specific Function:
- Receptor for platelet activating factor, a chemotactic phospholipid mediator that possesses potent inflammatory, smooth-muscle contractile and hypotensive activity. Seems to mediate its action via a G protein that activates a phosphatidylinositol-calcium second messenger system.
- Gene Name:
- PTAFR
- Uniprot ID:
- P25105
- Molecular Weight:
- 39203.075 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 2.61 uM | Not Available | BindingDB 50001728 |
| IC50 | 22 uM | Not Available | BindingDB 50001728 |
References
- Trova MP, Wissner A, Carroll ML, Kerwar SS, Pickett WC, Schaub RE, Torley LW, Kohler CA: Analogues of platelet activating factor. 8. Antagonists of PAF containing an aromatic ring linked to a pyridinium ring. J Med Chem. 1993 Mar 5;36(5):580-90. [8496938 ]
- Tahraoui L, Floch A, Cavero I: Functional validation of platelet-activating factor receptor sites characterized biochemically by a specific and reproducible [3H]platelet-activating factor binding in human platelets. J Pharmacol Exp Ther. 1990 Mar;252(3):1221-7. [2156995 ]
- General Function:
- Cholesterol binding
- Specific Function:
- Can bind protoporphyrin IX and may play a role in the transport of porphyrins and heme (By similarity). Promotes the transport of cholesterol across mitochondrial membranes and may play a role in lipid metabolism (PubMed:24814875), but its precise physiological role is controversial. It is apparently not required for steroid hormone biosynthesis. Was initially identified as peripheral-type benzodiazepine receptor; can also bind isoquinoline carboxamides (PubMed:1847678).
- Gene Name:
- TSPO
- Uniprot ID:
- P30536
- Molecular Weight:
- 18827.81 Da
References
- General Function:
- P53 binding
- Specific Function:
- Chromatin reader protein that recognizes and binds acetylated histones and plays a key role in transmission of epigenetic memory across cell divisions and transcription regulation. Remains associated with acetylated chromatin throughout the entire cell cycle and provides epigenetic memory for postmitotic G1 gene transcription by preserving acetylated chromatin status and maintaining high-order chromatin structure. During interphase, plays a key role in regulating the transcription of signal-inducible genes by associating with the P-TEFb complex and recruiting it to promoters: BRD4 is required to form the transcriptionally active P-TEFb complex by displacing negative regulators such as HEXIM1 and 7SKsnRNA complex from P-TEFb, thereby transforming it into an active form that can then phosphorylate the C-terminal domain (CTD) of RNA polymerase II. Promotes phosphorylation of 'Ser-2' of the C-terminal domain (CTD) of RNA polymerase II. According to a report, directly acts as an atypical protein kinase and mediates phosphorylation of 'Ser-2' of the C-terminal domain (CTD) of RNA polymerase II; these data however need additional evidences in vivo (PubMed:22509028). In addition to acetylated histones, also recognizes and binds acetylated RELA, leading to further recruitment of the P-TEFb complex and subsequent activation of NF-kappa-B. Also acts as a regulator of p53/TP53-mediated transcription: following phosphorylation by CK2, recruited to p53/TP53 specific target promoters.Isoform B: Acts as a chromatin insulator in the DNA damage response pathway. Inhibits DNA damage response signaling by recruiting the condensin-2 complex to acetylated histones, leading to chromatin structure remodeling, insulating the region from DNA damage response by limiting spreading of histone H2AFX/H2A.x phosphorylation.
- Gene Name:
- BRD4
- Uniprot ID:
- O60885
- Molecular Weight:
- 152217.955 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Dissociation | 2.46 uM | Not Available | BindingDB 50001728 |
References
- Filippakopoulos P, Picaud S, Fedorov O, Keller M, Wrobel M, Morgenstern O, Bracher F, Knapp S: Benzodiazepines and benzotriazepines as protein interaction inhibitors targeting bromodomains of the BET family. Bioorg Med Chem. 2012 Mar 15;20(6):1878-86. doi: 10.1016/j.bmc.2011.10.080. Epub 2011 Nov 4. [22137933 ]
- General Function:
- Type b gastrin/cholecystokinin receptor binding
- Specific Function:
- Receptor for gastrin and cholecystokinin. The CKK-B receptors occur throughout the central nervous system where they modulate anxiety, analgesia, arousal, and neuroleptic activity. This receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system.Isoform 2 is constitutively activated and may regulate cancer cell proliferation via a gastrin-independent mechanism.
- Gene Name:
- CCKBR
- Uniprot ID:
- P32239
- Molecular Weight:
- 48418.51 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >100 uM | Not Available | BindingDB 50001728 |
References
- Bock MG, DiPardo RM, Evans BE, Rittle KE, Veber DF, Freidinger RM, Chang RS, Lotti VJ: Cholecystokinin antagonists. Synthesis and biological evaluation of 4-substituted 4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepines. J Med Chem. 1988 Jan;31(1):176-81. [3336017 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRR3
- Uniprot ID:
- A8MPY1
- Molecular Weight:
- 54271.1 Da
References
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]