Estazolam (T3D3023)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:28:39 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:56 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D3023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Estazolam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Estazolam is only found in individuals that have used or taken this drug. It is a benzodiazepine with anticonvulsant, hypnotic, and muscle relaxant properties. It has been shown in some cases to be more potent than diazepam or nitrazepam. [PubChem] Benzodiazepines bind nonspecifically to benzodiazepine receptors, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
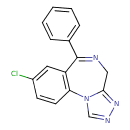
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C16H11ClN4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 294.738 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 294.067 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 29975-16-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 12-chloro-9-phenyl-2,4,5,8-tetraazatricyclo[8.4.0.0²,⁶]tetradeca-1(10),3,5,8,11,13-hexaene | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | estazolam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | ClC1=CC2=C(C=C1)N1C=NN=C1CN=C2C1=CC=CC=C1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C16H11ClN4/c17-12-6-7-14-13(8-12)16(11-4-2-1-3-5-11)18-9-15-20-19-10-21(14)15/h1-8,10H,9H2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=CDCHDCWJMGXXRH-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as 1,2,4-triazolo[4,3-a][1,4]benzodiazepines. These are aromatic compounds containing a 1,4-benzodiazepine fused to and sharing a nitrogen atom with a 1,2,4-triazole ring. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | 1,4-benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 1,2,4-triazolo[4,3-a][1,4]benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Tablets have been found to be equivalent in absorption to an orally administered solution of estazolam. In healthy subjects who received up to three times the recommended dose, peak estazolam plasma concentrations occurred within two hours after dosing (range 0.5 to 6.0 hours) and were proportional to the administered dose, suggesting linear pharmacokinetics over the dosage range tested. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Benzodiazepines bind nonspecifically to benzodiazepine receptors, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Extensively metabolized in the liver. In vitro studies with human liver microsomes indicate that the biotransformation of estazolam to the major circulating metabolite 4-hydroxy-estazolam is mediated by cytochrome P450 3A (CYP3A). Route of Elimination: Estazolam is extensively metabolized. The elimination of the parent drug takes place via hepatic metabolism of estazolam to hydroxylated and other metabolites that are eliminated largely in the urine both free and conjugated. Less than 5% of a 2 mg dose of estazolam was excreted unchanged in the urine, with only 4% of the dose appearing in the feces. Radiolabel mass balance studies indicate that the main route of excretion is via the kidneys. After 5 days, 87% of the administered radioactivity was excreted in human urine. Less than 4% of the dose was excreted unchanged. Half Life: The range of estimates for the mean elimination half-life of estazolam varies from 10 to 24 hours. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 740 mg/kg (oral, male mice) (4) LD50: 3200 mg/kg (oral, rat) (4) LD50 300 mg/kg (oral, rabbit) (4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For the short-term management of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | May cause a potentially dangerous rash that may develop into Stevens Johnson syndrome, an extremely rare but potentially fatal skin disease. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of overdose include confusion, depressed breathing, drowsiness and eventually coma, lack of coordination, and slurred speech. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Gastric evacuation, either by the induction of emesis, lavage, or both, should be performed immediately. Maintenance of adequate ventilation is essential. General supportive care, including frequent monitoring of the vital signs and close observation of the patient, is indicated. Fluids should be administered intravenously to maintain blood pressure and encourage diuresis. The value of dialysis in treatment of benzodiazepine overdose has not been determined. The physician may wish to consider contacting a Poison Control Center for up-to-date information on the management of hypnotic drug product overdose. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB01215 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB15346 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 3261 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL1201169 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 3146 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 4858 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Estazolam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Estazolam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Hester, J.B. Jr.; U.S. Patent 3,701,782; October 31, 1972; assigned to The Upjohn Co. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D3023.pdf | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA2
- Uniprot ID:
- P47869
- Molecular Weight:
- 51325.85 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA3
- Uniprot ID:
- P34903
- Molecular Weight:
- 55164.055 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Transporter activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA5
- Uniprot ID:
- P31644
- Molecular Weight:
- 52145.645 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRB1
- Uniprot ID:
- P18505
- Molecular Weight:
- 54234.085 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB2
- Uniprot ID:
- P47870
- Molecular Weight:
- 59149.895 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-gated chloride ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB3
- Uniprot ID:
- P28472
- Molecular Weight:
- 54115.04 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRD
- Uniprot ID:
- O14764
- Molecular Weight:
- 50707.835 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRE
- Uniprot ID:
- P78334
- Molecular Weight:
- 57971.175 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG1
- Uniprot ID:
- Q8N1C3
- Molecular Weight:
- 53594.49 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRG2
- Uniprot ID:
- P18507
- Molecular Weight:
- 54161.78 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG3
- Uniprot ID:
- Q99928
- Molecular Weight:
- 54288.16 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. In the uterus, the function of the receptor appears to be related to tissue contractility. The binding of this pI subunit with other GABA(A) receptor subunits alters the sensitivity of recombinant receptors to modulatory agents such as pregnanolone.
- Gene Name:
- GABRP
- Uniprot ID:
- O00591
- Molecular Weight:
- 50639.735 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-1 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR1
- Uniprot ID:
- P24046
- Molecular Weight:
- 55882.91 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-2 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR2
- Uniprot ID:
- P28476
- Molecular Weight:
- 54150.41 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRR3
- Uniprot ID:
- A8MPY1
- Molecular Weight:
- 54271.1 Da
References
- Braestrup C, Squires RF: Pharmacological characterization of benzodiazepine receptors in the brain. Eur J Pharmacol. 1978 Apr 1;48(3):263-70. [639854 ]
- Miller LG, Greenblatt DJ, Barnhill JG, Deutsch SI, Shader RI, Paul SM: Benzodiazepine receptor binding of triazolobenzodiazepines in vivo: increased receptor number with low-dose alprazolam. J Neurochem. 1987 Nov;49(5):1595-601. [2889803 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Cholesterol binding
- Specific Function:
- Can bind protoporphyrin IX and may play a role in the transport of porphyrins and heme (By similarity). Promotes the transport of cholesterol across mitochondrial membranes and may play a role in lipid metabolism (PubMed:24814875), but its precise physiological role is controversial. It is apparently not required for steroid hormone biosynthesis. Was initially identified as peripheral-type benzodiazepine receptor; can also bind isoquinoline carboxamides (PubMed:1847678).
- Gene Name:
- TSPO
- Uniprot ID:
- P30536
- Molecular Weight:
- 18827.81 Da
References
- Taketani S, Kohno H, Okuda M, Furukawa T, Tokunaga R: Induction of peripheral-type benzodiazepine receptors during differentiation of mouse erythroleukemia cells. A possible involvement of these receptors in heme biosynthesis. J Biol Chem. 1994 Mar 11;269(10):7527-31. [8125973 ]
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
18. GABA-A receptor (anion channel) (Protein Group)
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Included Proteins:
- P14867 , P47869 , P34903 , P48169 , P31644 , Q16445 , P18505 , P47870 , P28472 , O14764 , P78334 , Q8N1C3 , P18507 , Q99928 , O00591 , Q9UN88