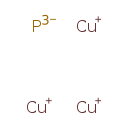Copper(I) phosphide (T3D1203)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-06-19 21:58:26 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:23:22 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D1203 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Copper(I) phosphide | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Copper(I) phosphide is a phosphide of copper. It is used in copper alloys. Copper is a chemical element with the symbol Cu and atomic number 29. Copper is an essential elements in plants and animals as it is required for the normal functioning of more than 30 enzymes. It occurs naturally throughout the environment in rocks, soil, water, and air. Metal phosphides are hydrolysed to phosphine upon contact with water or stomach acid. Phosphine is a colorless, flammable, explosive, and toxic gas. (13, 8, 9, 12) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | Cu3P | ||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 221.612 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 219.763 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 12019-57-7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | tris(λ¹-copper(1+) ion) phosphanetriide | ||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | tris(λ¹-copper(1+) ion) phosphanetriide | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [P-3].[Cu+].[Cu+].[Cu+] | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/3Cu.P/q3*+1;-3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=GKCDETHKBNXQFR-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as miscellaneous mixed metal/non-metals. These are inorganic compounds containing non-metal as well as metal atoms but not belonging to afore mentioned classes. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Mixed metal/non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Miscellaneous mixed metal/non-metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Miscellaneous mixed metal/non-metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Tan to reddish-brown solid. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral (8) ; inhalation (8) ; dermal (8) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Excess copper is sequestered within hepatocyte lysosomes, where it is complexed with metallothionein. Copper hepatotoxicity is believed to occur when the lysosomes become saturated and copper accumulates in the nucleus, causing nuclear damage. This damage is possibly a result of oxidative damage, including lipid peroxidation. Copper inhibits the sulfhydryl group enzymes such as glucose-6-phosphate 1-dehydrogenase, glutathione reductase, and paraoxonases, which protect the cell from free oxygen radicals. It also influences gene expression and is a co-factor for oxidative enzymes such as cytochrome C oxidase and lysyl oxidase. In addition, the oxidative stress induced by copper is thought to activate acid sphingomyelinase, which lead to the production of ceramide, an apoptotic signal, as well as cause hemolytic anemia. Copper-induced emesis results from stimulation of the vagus nerve. Phosphine inhibits cytochrome c oxidase, preventing mitochondrial oxidative phosphorylation. This non-competitive inhibition prevents cellular respiration and leads to multi-organ dysfunction. Phosphine can also react with hydrogen peroxide to form the highly reactive hydroxyl radical, which can cause lipid peroxidation. (3, 4, 8, 6, 1, 11) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Copper is mainly absorbed through the gastrointestinal tract, but it can also be inhalated and absorbed dermally. It passes through the basolateral membrane, possibly via regulatory copper transporters, and is transported to the liver and kidney bound to serum albumin. The liver is the critical organ for copper homoeostasis. In the liver and other tissues, copper is stored bound to metallothionein, amino acids, and in association with copper-dependent enzymes, then partitioned for excretion through the bile or incorporation into intra- and extracellular proteins. The transport of copper to the peripheral tissues is accomplished through the plasma attached to serum albumin, ceruloplasmin or low-molecular-weight complexes. Copper may induce the production of metallothionein and ceruloplasmin. The membrane-bound copper transporting adenosine triphosphatase (Cu-ATPase) transports copper ions into and out of cells. Physiologically normal levels of copper in the body are held constant by alterations in the rate and amount of copper absorption, compartmental distribution, and excretion. Phosphine and metal phosphides may be absorbed following ingestion or inhalation, then distribute to the nervous system, liver, and kidney. In the body, metal phosphides are hydrolysed to phosphine, and phosphine is oxidized to hypophosphite and phosphite. Metabolites are excreted in the urine, while unchanged phosphine is exhaled. (14, 8, 10) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | 10 to 20 grams for an adult human (copper salts). (5) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Copper(I) phosphide is used in copper alloys. (12) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Acute Oral: 0.01 mg/kg/day (7) Intermediate Oral: 0.01 mg/kg/day (7) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | People must absorb small amounts of copper every day because copper is essential for good health, however, high levels of copper can be harmful. Very-high doses of copper can cause damage to your liver and kidneys, and can even cause death. Copper may induce allergic responses in sensitive individuals. Inhalation of phosphine may cause severe pulmonary irritation leading to acute pulmonary oedema, cardiovascular dysfunction, CNS excitation, coma and death. Gastrointestinal disorders, renal damage and leukopenia may also occur. Chronic exposure to phosphine can result in anemia, bronchitis, gastrointestinal effects, and visual, speech and motor problems. (13, 14, 9, 10) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Breathing high levels of copper can cause irritation of the nose and throat. Ingesting high levels of copper can cause nausea, vomiting, diarrhea, headache, dizziness, and respiratory difficulty. Early symptoms of acute phosphine intoxication include pain in the diaphragm, nausea, vomiting, excitement, and a phosphorus smell on the breath. Higher levels can cause weakness, bronchitis, pulmonary edema, shortness of breath, convulsions, and death. Some effects, such as pulmonary edema, convulsions, and liver injury, may appear or continue to be present days after an exposure. Ingestion of metal phosphides results in release of phosphine in your stomach which can cause nausea, vomiting, abdominal pain, and diarrhea. (13, 9, 10) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | As there is no antidote for phosphine poisoning, treatment is mainly symptomatic. Artificial respiration, gastric lavage, and/or administration of activated charcoal may be necessary. (14) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 159399 | ||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Copper(I) phosphide | ||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Protein homodimerization activity
- Specific Function:
- Catalyzes the rate-limiting step of the oxidative pentose-phosphate pathway, which represents a route for the dissimilation of carbohydrates besides glycolysis. The main function of this enzyme is to provide reducing power (NADPH) and pentose phosphates for fatty acid and nucleic acid synthesis.
- Gene Name:
- G6PD
- Uniprot ID:
- P11413
- Molecular Weight:
- 59256.31 Da
References
- Brewer GJ: A brand new mechanism for copper toxicity. J Hepatol. 2007 Oct;47(4):621-2. Epub 2007 Jul 23. [17697726 ]
- Baxter PJ, Adams PH, & Aw TC (2000). Hunter's Diseases of Occupations. 9th ed. New York, NY: Oxford University Press Inc.
- Wikipedia. Copper. Last Updated 29 May 2009. [Link]
- US Environmental Protection Agency (2008). Drinking Water Health Advisory for 2,4-Dinitrotoluene and 2,6-Dinitrotoluene. [Link]
- General Function:
- Nadp binding
- Specific Function:
- Maintains high levels of reduced glutathione in the cytosol.
- Gene Name:
- GSR
- Uniprot ID:
- P00390
- Molecular Weight:
- 56256.565 Da
References
- Brewer GJ: A brand new mechanism for copper toxicity. J Hepatol. 2007 Oct;47(4):621-2. Epub 2007 Jul 23. [17697726 ]
- Baxter PJ, Adams PH, & Aw TC (2000). Hunter's Diseases of Occupations. 9th ed. New York, NY: Oxford University Press Inc.
- Wikipedia. Copper. Last Updated 29 May 2009. [Link]
- US Environmental Protection Agency (2008). Drinking Water Health Advisory for 2,4-Dinitrotoluene and 2,6-Dinitrotoluene. [Link]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Hydrolyzes the toxic metabolites of a variety of organophosphorus insecticides. Capable of hydrolyzing a broad spectrum of organophosphate substrates and lactones, and a number of aromatic carboxylic acid esters. Mediates an enzymatic protection of low density lipoproteins against oxidative modification and the consequent series of events leading to atheroma formation.
- Gene Name:
- PON1
- Uniprot ID:
- P27169
- Molecular Weight:
- 39730.99 Da
References
- Brewer GJ: A brand new mechanism for copper toxicity. J Hepatol. 2007 Oct;47(4):621-2. Epub 2007 Jul 23. [17697726 ]
- Baxter PJ, Adams PH, & Aw TC (2000). Hunter's Diseases of Occupations. 9th ed. New York, NY: Oxford University Press Inc.
- Wikipedia. Copper. Last Updated 29 May 2009. [Link]
- US Environmental Protection Agency (2008). Drinking Water Health Advisory for 2,4-Dinitrotoluene and 2,6-Dinitrotoluene. [Link]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Has low activity towards the organophosphate paraxon and aromatic carboxylic acid esters. Rapidly hydrolyzes lactones such as statin prodrugs (e.g. lovastatin). Hydrolyzes aromatic lactones and 5- or 6-member ring lactones with aliphatic substituents but not simple lactones or those with polar substituents.
- Gene Name:
- PON3
- Uniprot ID:
- Q15166
- Molecular Weight:
- 39607.185 Da
References
- Brewer GJ: A brand new mechanism for copper toxicity. J Hepatol. 2007 Oct;47(4):621-2. Epub 2007 Jul 23. [17697726 ]
- Baxter PJ, Adams PH, & Aw TC (2000). Hunter's Diseases of Occupations. 9th ed. New York, NY: Oxford University Press Inc.
- Wikipedia. Copper. Last Updated 29 May 2009. [Link]
- US Environmental Protection Agency (2008). Drinking Water Health Advisory for 2,4-Dinitrotoluene and 2,6-Dinitrotoluene. [Link]
- General Function:
- Not Available
- Specific Function:
- Not Available
- Gene Name:
- SNCA
- Uniprot ID:
- P37840
- Molecular Weight:
- 14460.155 Da
References
- Davies P, Fontaine SN, Moualla D, Wang X, Wright JA, Brown DR: Amyloidogenic metal-binding proteins: new investigative pathways. Biochem Soc Trans. 2008 Dec;36(Pt 6):1299-303. doi: 10.1042/BST0361299. [19021544 ]
- General Function:
- Transition metal ion binding
- Specific Function:
- Functions as a cell surface receptor and performs physiological functions on the surface of neurons relevant to neurite growth, neuronal adhesion and axonogenesis. Involved in cell mobility and transcription regulation through protein-protein interactions. Can promote transcription activation through binding to APBB1-KAT5 and inhibits Notch signaling through interaction with Numb. Couples to apoptosis-inducing pathways such as those mediated by G(O) and JIP. Inhibits G(o) alpha ATPase activity (By similarity). Acts as a kinesin I membrane receptor, mediating the axonal transport of beta-secretase and presenilin 1. Involved in copper homeostasis/oxidative stress through copper ion reduction. In vitro, copper-metallated APP induces neuronal death directly or is potentiated through Cu(2+)-mediated low-density lipoprotein oxidation. Can regulate neurite outgrowth through binding to components of the extracellular matrix such as heparin and collagen I and IV. The splice isoforms that contain the BPTI domain possess protease inhibitor activity. Induces a AGER-dependent pathway that involves activation of p38 MAPK, resulting in internalization of amyloid-beta peptide and leading to mitochondrial dysfunction in cultured cortical neurons. Provides Cu(2+) ions for GPC1 which are required for release of nitric oxide (NO) and subsequent degradation of the heparan sulfate chains on GPC1.Beta-amyloid peptides are lipophilic metal chelators with metal-reducing activity. Bind transient metals such as copper, zinc and iron. In vitro, can reduce Cu(2+) and Fe(3+) to Cu(+) and Fe(2+), respectively. Beta-amyloid 42 is a more effective reductant than beta-amyloid 40. Beta-amyloid peptides bind to lipoproteins and apolipoproteins E and J in the CSF and to HDL particles in plasma, inhibiting metal-catalyzed oxidation of lipoproteins. Beta-APP42 may activate mononuclear phagocytes in the brain and elicit inflammatory responses. Promotes both tau aggregation and TPK II-mediated phosphorylation. Interaction with overexpressed HADH2 leads to oxidative stress and neurotoxicity. Also binds GPC1 in lipid rafts.Appicans elicit adhesion of neural cells to the extracellular matrix and may regulate neurite outgrowth in the brain.The gamma-CTF peptides as well as the caspase-cleaved peptides, including C31, are potent enhancers of neuronal apoptosis.N-APP binds TNFRSF21 triggering caspase activation and degeneration of both neuronal cell bodies (via caspase-3) and axons (via caspase-6).
- Gene Name:
- APP
- Uniprot ID:
- P05067
- Molecular Weight:
- 86942.715 Da
References
- Davies P, Fontaine SN, Moualla D, Wang X, Wright JA, Brown DR: Amyloidogenic metal-binding proteins: new investigative pathways. Biochem Soc Trans. 2008 Dec;36(Pt 6):1299-303. doi: 10.1042/BST0361299. [19021544 ]
- General Function:
- Iron ion binding
- Specific Function:
- Cytochrome c oxidase is the component of the respiratory chain that catalyzes the reduction of oxygen to water. Subunits 1-3 form the functional core of the enzyme complex. CO I is the catalytic subunit of the enzyme. Electrons originating in cytochrome c are transferred via the copper A center of subunit 2 and heme A of subunit 1 to the bimetallic center formed by heme A3 and copper B.
- Gene Name:
- MT-CO1
- Uniprot ID:
- P00395
- Molecular Weight:
- 57040.91 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- Cytochrome c oxidase is the component of the respiratory chain that catalyzes the reduction of oxygen to water. Subunits 1-3 form the functional core of the enzyme complex. Subunit 2 transfers the electrons from cytochrome c via its binuclear copper A center to the bimetallic center of the catalytic subunit 1.
- Gene Name:
- MT-CO2
- Uniprot ID:
- P00403
- Molecular Weight:
- 25564.73 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- Subunits I, II and III form the functional core of the enzyme complex.
- Gene Name:
- MT-CO3
- Uniprot ID:
- P00414
- Molecular Weight:
- 29950.6 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX4I1
- Uniprot ID:
- P13073
- Molecular Weight:
- 19576.6 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX4I2
- Uniprot ID:
- Q96KJ9
- Molecular Weight:
- 20010.02 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Metal ion binding
- Specific Function:
- This is the heme A-containing chain of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX5A
- Uniprot ID:
- P20674
- Molecular Weight:
- 16761.985 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Metal ion binding
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX5B
- Uniprot ID:
- P10606
- Molecular Weight:
- 13695.57 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6A1
- Uniprot ID:
- P12074
- Molecular Weight:
- 12154.8 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6A2
- Uniprot ID:
- Q02221
- Molecular Weight:
- 10815.32 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- Connects the two COX monomers into the physiological dimeric form.
- Gene Name:
- COX6B1
- Uniprot ID:
- P14854
- Molecular Weight:
- 10192.345 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- Connects the two COX monomers into the physiological dimeric form.
- Gene Name:
- COX6B2
- Uniprot ID:
- Q6YFQ2
- Molecular Weight:
- 10528.905 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6C
- Uniprot ID:
- P09669
- Molecular Weight:
- 8781.36 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7A1
- Uniprot ID:
- P24310
- Molecular Weight:
- 9117.44 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7A2
- Uniprot ID:
- P14406
- Molecular Weight:
- 9395.89 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport. Plays a role in proper central nervous system (CNS) development in vertebrates.
- Gene Name:
- COX7B
- Uniprot ID:
- P24311
- Molecular Weight:
- 9160.485 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7B2
- Uniprot ID:
- Q8TF08
- Molecular Weight:
- 9077.43 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7C
- Uniprot ID:
- P15954
- Molecular Weight:
- 7245.45 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX8A
- Uniprot ID:
- P10176
- Molecular Weight:
- 7579.0 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX8C
- Uniprot ID:
- Q7Z4L0
- Molecular Weight:
- 8128.575 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- Not Available
- Gene Name:
- COX7A2P2
- Uniprot ID:
- O60397
- Molecular Weight:
- 11840.715 Da
References
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K: Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006;44(2):155-8. [16615671 ]