You are using an unsupported browser. Please upgrade your browser to a newer version to get the best experience on Toxin, Toxin Target Database.
Risperidone (T3D2871)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:27:30 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:52 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2871 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Risperidone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Risperidone is an atypical antipsychotic medication approved in 1993. It is most often used to treat delusional psychosis (including schizophrenia), but risperidone (like other atypical antipsychotics) is also used to treat some forms of bipolar disorder, psychotic depression and Tourette syndrome. Generally lower doses are used for autistic spectrum disorders than are used for schizophrenia and other forms of psychosis; Risperidone is a very strong dopamine blocker (antagonist); Risperidone is a very strong dopamine blocker (antagonist); i.e., it inhibits functioning of postsynaptic dopamine receptors. An anxiolytic agent and a serotonin receptor agonist belonging to the azaspirodecanedione class of compounds. Its structure is unrelated to those of the benzodiazepines, but it has an efficacy comparable to diazepam; i.e., it inhibits functioning of postsynaptic dopamine receptors. Risperidone (Belivon, Rispen, Risperdal; in the United States) is an atypical antipsychotic medication. It was approved by the United States Food and Drug Administration (FDA) in 1993. It is most often used to treat delusional psychosis (including schizophrenia), but risperidone (like other atypical antipsychotics) is also used to treat some forms of bipolar disorder, psychotic depression and Tourette syndrome; risperidone has received approval from the Food and Drug Administration (FDA) for symptomatic treatment of irritability in autistic children and adolescents. Risperidone is now the most commonly prescribed antipsychotic medication in the United States. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
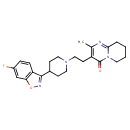
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C23H27FN4O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 410.485 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 410.212 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 106266-06-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 3-{2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-4H,6H,7H,8H,9H-pyrido[1,2-a]pyrimidin-4-one | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | risperidone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CC1=C(CCN2CCC(CC2)C2=NOC3=C2C=CC(F)=C3)C(=O)N2CCCCC2=N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=RAPZEAPATHNIPO-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as pyridopyrimidines. Pyridopyrimidines are compounds containing a pyridopyrimidine, which consists of a pyridine fused to a pyrimidine. Pyridine is 6-membered ring consisting of five carbon atoms and a nitrogen atom. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyridopyrimidines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyridopyrimidines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Blockade of dopaminergic D2 receptors in the limbic system alleviates positive symptoms of schizophrenia such as hallucinations, delusions, and erratic behavior and speech. Blockade of serotonergic 5-HT2 receptors in the mesocortical tract, causes an excess of dopamine and an increase in dopamine transmission, resulting in an increase in dopamine transmission and an elimination of core negative symptoms. Dopamine receptors in the nigrostriatal pathway are not affected by risperidone and extrapyramidal effects are avoided. Like other 5-HT2 antagonists, risperidone also binds at alpha(1)-adrenergic receptors and, to a lesser extent, at histamine H1 and alpha(2)-adrenergic receptors. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Extensively metabolized by hepatic cytochrome P450 2D6 isozyme to 9-hydroxyrisperidone, which has approximately the same receptor binding affinity as risperidone. Hydroxylation is dependent on debrisoquine 4-hydroxylase and metabolism is sensitive to genetic polymorphisms in debrisoquine 4-hydroxylase. Risperidone also undergoes N-dealkylation to a lesser extent. Route of Elimination: Risperidone is extensively metabolized in the liver.In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives were prolonged compared to young healthy subjects. Half Life: 20-24 hours | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50=82.1mg/kg (orally in mice). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For the treatment of schizophrenia in adults and in adolescents, ages 13 to 17, and for the short-term treatment of manic or mixed episodes of bipolar I disorder in children and adolescents ages 10 to 17. May also be used to manage symptoms of inappropriate behavior due to aggression and/or psychosis in patients with severe dementia. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of overdose include drowsiness, sedation, tachycardia, hypotension, and extrapyramidal symptoms. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | In case of acute overdosage, establish and maintain an airway and ensure adequate oxygenation and ventilation. Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. The possibility of obtundation, seizures, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine carry a theoretical hazard of QT-prolonging effects that might be additive to those of risperidone. Similarly, it is reasonable to expect that the alpha-blocking properties of bretylium might be additive to those of risperidone, resulting in problematic hypotension. There is no specific antidote to Risperidone. (9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00734 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB05020 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 5073 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 4895 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C06861 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | 608902 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 8871 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Risperidone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Risperidone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Virus receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including mescaline, psilocybin, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates phospholipase C and a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and promotes the release of Ca(2+) ions from intracellular stores. Affects neural activity, perception, cognition and mood. Plays a role in the regulation of behavior, including responses to anxiogenic situations and psychoactive substances. Plays a role in intestinal smooth muscle contraction, and may play a role in arterial vasoconstriction.(Microbial infection) Acts as a receptor for human JC polyomavirus/JCPyV.
- Gene Name:
- HTR2A
- Uniprot ID:
- P28223
- Molecular Weight:
- 52602.58 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.00016 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.00029 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.00039 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.00042 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.00043 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.00052 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.00081 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0011 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.007 uM | Not Available | BindingDB 50001885 |
References
- McDonald LM, Moran PM, Vythelingum GN, Joseph MH, Stephenson JD, Gray JA: Enhancement of latent inhibition by two 5-HT2A receptor antagonists only when given at both pre-exposure and conditioning. Psychopharmacology (Berl). 2003 Sep;169(3-4):321-31. Epub 2002 Aug 9. [14530903 ]
- Keks NA, Culhane C: Risperidone (Risperdal): clinical experience with a new antipsychosis drug. Expert Opin Investig Drugs. 1999 Apr;8(4):443-52. [15992090 ]
- Reimold M, Solbach C, Noda S, Schaefer JE, Bartels M, Beneke M, Machulla HJ, Bares R, Glaser T, Wormstall H: Occupancy of dopamine D(1), D (2) and serotonin (2A) receptors in schizophrenic patients treated with flupentixol in comparison with risperidone and haloperidol. Psychopharmacology (Berl). 2007 Feb;190(2):241-9. Epub 2006 Nov 17. [17111172 ]
- Slifstein M, Kegeles LS, Gonzales R, Frankle WG, Xu X, Laruelle M, Abi-Dargham A: [11C]NNC 112 selectivity for dopamine D1 and serotonin 5-HT(2A) receptors: a PET study in healthy human subjects. J Cereb Blood Flow Metab. 2007 Oct;27(10):1733-41. Epub 2007 Feb 21. [17311076 ]
- Uchida S, Kato Y, Hirano K, Kagawa Y, Yamada S: Brain neurotransmitter receptor-binding characteristics in rats after oral administration of haloperidol, risperidone and olanzapine. Life Sci. 2007 Apr 3;80(17):1635-40. Epub 2007 Jan 27. [17316700 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL: Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther. 1996 Feb;276(2):720-7. [8632342 ]
- Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994 May;55 Suppl:5-12. [7520908 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Kongsamut S, Roehr JE, Cai J, Hartman HB, Weissensee P, Kerman LL, Tang L, Sandrasagra A: Iloperidone binding to human and rat dopamine and 5-HT receptors. Eur J Pharmacol. 1996 Dec 19;317(2-3):417-23. [8997630 ]
- Kongsamut S, Kang J, Chen XL, Roehr J, Rampe D: A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. Eur J Pharmacol. 2002 Aug 16;450(1):37-41. [12176106 ]
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO: Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res. 1999 May 4;37(1):107-22. [10227113 ]
- Davies MA, Setola V, Strachan RT, Sheffler DJ, Salay E, Hufeisen SJ, Roth BL: Pharmacologic analysis of non-synonymous coding h5-HT2A SNPs reveals alterations in atypical antipsychotic and agonist efficacies. Pharmacogenomics J. 2006 Jan-Feb;6(1):42-51. [16314884 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including ergot alkaloid derivatives, 1-2,5,-dimethoxy-4-iodophenyl-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Regulates neuronal activity via the activation of short transient receptor potential calcium channels in the brain, and thereby modulates the activation of pro-opiomelacortin neurons and the release of CRH that then regulates the release of corticosterone. Plays a role in the regulation of appetite and eating behavior, responses to anxiogenic stimuli and stress. Plays a role in insulin sensitivity and glucose homeostasis.
- Gene Name:
- HTR2C
- Uniprot ID:
- P28335
- Molecular Weight:
- 51820.705 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0064 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.01 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.012 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.024 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.026 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.033 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.044 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.063 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.064 uM | Not Available | BindingDB 50001885 |
References
- Richelson E, Souder T: Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000 Nov 24;68(1):29-39. [11132243 ]
- Canton H, Verriele L, Millan MJ: Competitive antagonism of serotonin (5-HT)2C and 5-HT2A receptor-mediated phosphoinositide (PI) turnover by clozapine in the rat: a comparison to other antipsychotics. Neurosci Lett. 1994 Nov 7;181(1-2):65-8. [7898773 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Kongsamut S, Roehr JE, Cai J, Hartman HB, Weissensee P, Kerman LL, Tang L, Sandrasagra A: Iloperidone binding to human and rat dopamine and 5-HT receptors. Eur J Pharmacol. 1996 Dec 19;317(2-3):417-23. [8997630 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Herrick-Davis K, Grinde E, Teitler M: Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther. 2000 Oct;295(1):226-32. [10991983 ]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT: Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996 Feb;14(2):87-96. [8822531 ]
- Krogsgaard-Larsen N, Jensen AA, Kehler J: Novel 7-phenylsulfanyl-1,2,3,4,10,10a-hexahydro-pyrazino[1,2-a]indoles as dual serotonin 5-HT2C and 5-HT6 receptor ligands. Bioorg Med Chem Lett. 2010 Sep 15;20(18):5431-3. doi: 10.1016/j.bmcl.2010.07.105. Epub 2010 Aug 3. [20719507 ]
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO: Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res. 1999 May 4;37(1):107-22. [10227113 ]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL: Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Oct;27(7):1125-43. [14642972 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL: Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther. 1996 Feb;276(2):720-7. [8632342 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which inhibit adenylyl cyclase.
- Gene Name:
- DRD2
- Uniprot ID:
- P14416
- Molecular Weight:
- 50618.91 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.00044 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0014 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0022 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0027 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0033 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0064 uM | Not Available | BindingDB 50001885 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Naiker DV, Catts SV, Catts VS, Bedi KS, Bryan-Lluka LJ: Dose determination of haloperidol, risperidone and olanzapine using an in vivo dopamine D2-receptor occupancy method in the rat. Eur J Pharmacol. 2006 Jul 1;540(1-3):87-90. Epub 2006 May 11. [16730699 ]
- Remington G, Mamo D, Labelle A, Reiss J, Shammi C, Mannaert E, Mann S, Kapur S: A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006 Mar;163(3):396-401. [16513859 ]
- Lane HY, Lee CC, Chang YC, Lu CT, Huang CH, Chang WH: Effects of dopamine D2 receptor Ser311Cys polymorphism and clinical factors on risperidone efficacy for positive and negative symptoms and social function. Int J Neuropsychopharmacol. 2004 Dec;7(4):461-70. Epub 2004 May 12. [15140279 ]
- Catafau AM, Corripio I, Perez V, Martin JC, Schotte A, Carrio I, Alvarez E: Dopamine D2 receptor occupancy by risperidone: implications for the timing and magnitude of clinical response. Psychiatry Res. 2006 Dec 1;148(2-3):175-83. Epub 2006 Oct 23. [17059881 ]
- Yamanouchi Y, Iwata N, Suzuki T, Kitajima T, Ikeda M, Ozaki N: Effect of DRD2, 5-HT2A, and COMT genes on antipsychotic response to risperidone. Pharmacogenomics J. 2003;3(6):356-61. [14610521 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Phillips ST, de Paulis T, Neergaard JR, Baron BM, Siegel BW, Seeman P, Van Tol HH, Guan HC, Smith HE: Binding of 5H-dibenzo[a,d]cycloheptene and dibenz[b,f]oxepin analogues of clozapine to dopamine and serotonin receptors. J Med Chem. 1995 Feb 17;38(4):708-14. [7861418 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Mattsson C, Andreasson T, Waters N, Sonesson C: Systematic in vivo screening of a series of 1-propyl-4-arylpiperidines against dopaminergic and serotonergic properties in rat brain: a scaffold-jumping approach. J Med Chem. 2012 Nov 26;55(22):9735-50. doi: 10.1021/jm300975f. Epub 2012 Oct 17. [23043306 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Uchida H, Rajji TK, Mulsant BH, Kapur S, Pollock BG, Graff-Guerrero A, Menon M, Mamo DC: D2 receptor blockade by risperidone correlates with attention deficits in late-life schizophrenia. J Clin Psychopharmacol. 2009 Dec;29(6):571-5. doi: 10.1097/JCP.0b013e3181bf4ea3. [19910723 ]
- General Function:
- Histamine receptor activity
- Specific Function:
- In peripheral tissues, the H1 subclass of histamine receptors mediates the contraction of smooth muscles, increase in capillary permeability due to contraction of terminal venules, and catecholamine release from adrenal medulla, as well as mediating neurotransmission in the central nervous system.
- Gene Name:
- HRH1
- Uniprot ID:
- P35367
- Molecular Weight:
- 55783.61 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0026 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.019 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.033 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.088 uM | Not Available | BindingDB 50001885 |
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Richelson E, Souder T: Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000 Nov 24;68(1):29-39. [11132243 ]
- Schreiber S, Backer MM, Weizman R, Pick CG: Augmentation of opioid induced antinociception by the atypical antipsychotic drug risperidone in mice. Neurosci Lett. 1997 May 30;228(1):25-8. [9197279 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which inhibit adenylyl cyclase. Promotes cell proliferation.
- Gene Name:
- DRD3
- Uniprot ID:
- P35462
- Molecular Weight:
- 44224.335 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0067 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0096 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.013 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.014 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.016 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.031 uM | Not Available | BindingDB 50001885 |
References
- Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994 May;55 Suppl:5-12. [7520908 ]
- Megens AA, Awouters FH, Schotte A, Meert TF, Dugovic C, Niemegeers CJ, Leysen JE: Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology (Berl). 1994 Feb;114(1):9-23. [7531353 ]
- Butini S, Gemma S, Campiani G, Franceschini S, Trotta F, Borriello M, Ceres N, Ros S, Coccone SS, Bernetti M, De Angelis M, Brindisi M, Nacci V, Fiorini I, Novellino E, Cagnotto A, Mennini T, Sandager-Nielsen K, Andreasen JT, Scheel-Kruger J, Mikkelsen JD, Fattorusso C: Discovery of a new class of potential multifunctional atypical antipsychotic agents targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors: design, synthesis, and effects on behavior. J Med Chem. 2009 Jan 8;52(1):151-69. doi: 10.1021/jm800689g. [19072656 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Bolos J, Anglada L, Gubert S, Planas JM, Agut J, Princep M, De la Fuente N, Sacristan A, Ortiz JA: 7-[3-(1-piperidinyl)propoxy]chromenones as potential atypical antipsychotics. 2. Pharmacological profile of 7-[3-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)-piperidin-1-yl]propoxy]-3-(hydroxymeth yl)chromen -4-one (abaperidone, FI-8602). J Med Chem. 1998 Dec 31;41(27):5402-9. [9876110 ]
- General Function:
- Sh3 domain binding
- Specific Function:
- Dopamine receptor responsible for neuronal signaling in the mesolimbic system of the brain, an area of the brain that regulates emotion and complex behavior. Its activity is mediated by G proteins which inhibit adenylyl cyclase. Modulates the circadian rhythm of contrast sensitivity by regulating the rhythmic expression of NPAS2 in the retinal ganglion cells (By similarity).
- Gene Name:
- DRD4
- Uniprot ID:
- P21917
- Molecular Weight:
- 48359.86 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0062 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.007 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0085 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.016 uM | Not Available | BindingDB 50001885 |
References
- Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994 May;55 Suppl:5-12. [7520908 ]
- Megens AA, Awouters FH, Schotte A, Meert TF, Dugovic C, Niemegeers CJ, Leysen JE: Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology (Berl). 1994 Feb;114(1):9-23. [7531353 ]
- Bolos J, Anglada L, Gubert S, Planas JM, Agut J, Princep M, De la Fuente N, Sacristan A, Ortiz JA: 7-[3-(1-piperidinyl)propoxy]chromenones as potential atypical antipsychotics. 2. Pharmacological profile of 7-[3-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)-piperidin-1-yl]propoxy]-3-(hydroxymeth yl)chromen -4-one (abaperidone, FI-8602). J Med Chem. 1998 Dec 31;41(27):5402-9. [9876110 ]
- Phillips ST, de Paulis T, Neergaard JR, Baron BM, Siegel BW, Seeman P, Van Tol HH, Guan HC, Smith HE: Binding of 5H-dibenzo[a,d]cycloheptene and dibenz[b,f]oxepin analogues of clozapine to dopamine and serotonin receptors. J Med Chem. 1995 Feb 17;38(4):708-14. [7861418 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- General Function:
- Voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization
- Specific Function:
- Pore-forming (alpha) subunit of voltage-gated inwardly rectifying potassium channel. Channel properties are modulated by cAMP and subunit assembly. Mediates the rapidly activating component of the delayed rectifying potassium current in heart (IKr). Isoforms USO have no channel activity by themself, but modulates channel characteristics by forming heterotetramers with other isoforms which are retained intracellularly and undergo ubiquitin-dependent degradation.
- Gene Name:
- KCNH2
- Uniprot ID:
- Q12809
- Molecular Weight:
- 126653.52 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.92 uM | Not Available | BindingDB 50001885 |
| IC50 | 0.151 uM | Not Available | BindingDB 50001885 |
| IC50 | 0.15136 uM | Not Available | BindingDB 50001885 |
| IC50 | 0.162 uM | Not Available | BindingDB 50001885 |
| IC50 | 0.16218 uM | Not Available | BindingDB 50001885 |
| IC50 | 0.163 uM | Not Available | BindingDB 50001885 |
| IC50 | 0.26 uM | Not Available | BindingDB 50001885 |
References
- Keseru GM: Prediction of hERG potassium channel affinity by traditional and hologram qSAR methods. Bioorg Med Chem Lett. 2003 Aug 18;13(16):2773-5. [12873512 ]
- Jia L, Sun H: Support vector machines classification of hERG liabilities based on atom types. Bioorg Med Chem. 2008 Jun 1;16(11):6252-60. doi: 10.1016/j.bmc.2008.04.028. Epub 2008 Apr 16. [18448342 ]
- Rajamani R, Tounge BA, Li J, Reynolds CH: A two-state homology model of the hERG K+ channel: application to ligand binding. Bioorg Med Chem Lett. 2005 Mar 15;15(6):1737-41. [15745831 ]
- Ermondi G, Visentin S, Caron G: GRIND-based 3D-QSAR and CoMFA to investigate topics dominated by hydrophobic interactions: the case of hERG K+ channel blockers. Eur J Med Chem. 2009 May;44(5):1926-32. doi: 10.1016/j.ejmech.2008.11.009. Epub 2008 Nov 28. [19110341 ]
- Cavalli A, Poluzzi E, De Ponti F, Recanatini M: Toward a pharmacophore for drugs inducing the long QT syndrome: insights from a CoMFA study of HERG K(+) channel blockers. J Med Chem. 2002 Aug 29;45(18):3844-53. [12190308 ]
- Du LP, Tsai KC, Li MY, You QD, Xia L: The pharmacophore hypotheses of I(Kr) potassium channel blockers: novel class III antiarrhythmic agents. Bioorg Med Chem Lett. 2004 Sep 20;14(18):4771-7. [15324906 ]
- Butini S, Gemma S, Campiani G, Franceschini S, Trotta F, Borriello M, Ceres N, Ros S, Coccone SS, Bernetti M, De Angelis M, Brindisi M, Nacci V, Fiorini I, Novellino E, Cagnotto A, Mennini T, Sandager-Nielsen K, Andreasen JT, Scheel-Kruger J, Mikkelsen JD, Fattorusso C: Discovery of a new class of potential multifunctional atypical antipsychotic agents targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors: design, synthesis, and effects on behavior. J Med Chem. 2009 Jan 8;52(1):151-69. doi: 10.1021/jm800689g. [19072656 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- This alpha-adrenergic receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Its effect is mediated by G(q) and G(11) proteins. Nuclear ADRA1A-ADRA1B heterooligomers regulate phenylephrine(PE)-stimulated ERK signaling in cardiac myocytes.
- Gene Name:
- ADRA1A
- Uniprot ID:
- P35348
- Molecular Weight:
- 51486.005 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0028 uM | Not Available | BindingDB 50001885 |
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Sleight AJ, Koek W, Bigg DC: Binding of antipsychotic drugs at alpha 1A- and alpha 1B-adrenoceptors: risperidone is selective for the alpha 1B-adrenoceptors. Eur J Pharmacol. 1993 Jul 20;238(2-3):407-10. [7691623 ]
- Eltze M: In functional experiments, risperidone is selective, not for the B, but for the A subtype of alpha 1-adrenoceptors. Eur J Pharmacol. 1996 Jan 4;295(1):69-73. [8925876 ]
- Marek GJ, Aghajanian GK: Alpha 1B-adrenoceptor-mediated excitation of piriform cortical interneurons. Eur J Pharmacol. 1996 Jun 3;305(1-3):95-100. [8813537 ]
- Li MY, Tsai KC, Xia L: Pharmacophore identification of alpha(1A)-adrenoceptor antagonists. Bioorg Med Chem Lett. 2005 Feb 1;15(3):657-64. [15664832 ]
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which activate adenylyl cyclase.
- Gene Name:
- DRD1
- Uniprot ID:
- P21728
- Molecular Weight:
- 49292.765 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.021 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.523 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.58 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.62 uM | Not Available | BindingDB 50001885 |
References
- Keks NA, Culhane C: Risperidone (Risperdal): clinical experience with a new antipsychosis drug. Expert Opin Investig Drugs. 1999 Apr;8(4):443-52. [15992090 ]
- Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994 May;55 Suppl:5-12. [7520908 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Kongsamut S, Roehr JE, Cai J, Hartman HB, Weissensee P, Kerman LL, Tang L, Sandrasagra A: Iloperidone binding to human and rat dopamine and 5-HT receptors. Eur J Pharmacol. 1996 Dec 19;317(2-3):417-23. [8997630 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Gene Name:
- HTR1A
- Uniprot ID:
- P08908
- Molecular Weight:
- 46106.335 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.021 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.21 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.417 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.42 uM | Not Available | BindingDB 50001885 |
References
- Richelson E, Souder T: Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000 Nov 24;68(1):29-39. [11132243 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994 May;55 Suppl:5-12. [7520908 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that stimulate adenylate cyclase. It has a high affinity for tricyclic psychotropic drugs (By similarity). Controls pyramidal neurons migration during corticogenesis, through the regulation of CDK5 activity (By similarity). Is an activator of TOR signaling (PubMed:23027611).
- Gene Name:
- HTR6
- Uniprot ID:
- P50406
- Molecular Weight:
- 46953.625 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.224 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 2 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 2.4 uM | Not Available | BindingDB 50001885 |
References
- Krogsgaard-Larsen N, Jensen AA, Kehler J: Novel 7-phenylsulfanyl-1,2,3,4,10,10a-hexahydro-pyrazino[1,2-a]indoles as dual serotonin 5-HT2C and 5-HT6 receptor ligands. Bioorg Med Chem Lett. 2010 Sep 15;20(18):5431-3. doi: 10.1016/j.bmcl.2010.07.105. Epub 2010 Aug 3. [20719507 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL, Hamblin MW: Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J Neurochem. 1996 Jan;66(1):47-56. [8522988 ]
- Arnt J, Skarsfeldt T: Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998 Feb;18(2):63-101. [9430133 ]
- General Function:
- Thioesterase binding
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins. The rank order of potency for agonists of this receptor is oxymetazoline > clonidine > epinephrine > norepinephrine > phenylephrine > dopamine > p-synephrine > p-tyramine > serotonin = p-octopamine. For antagonists, the rank order is yohimbine > phentolamine = mianserine > chlorpromazine = spiperone = prazosin > propanolol > alprenolol = pindolol.
- Gene Name:
- ADRA2A
- Uniprot ID:
- P08913
- Molecular Weight:
- 48956.275 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.01 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.023 uM | Not Available | BindingDB 50001885 |
References
- Fenton C, Scott LJ: Risperidone: a review of its use in the treatment of bipolar mania. CNS Drugs. 2005;19(5):429-44. [15907153 ]
- Richelson E, Souder T: Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000 Nov 24;68(1):29-39. [11132243 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins.
- Gene Name:
- ADRA2C
- Uniprot ID:
- P18825
- Molecular Weight:
- 49521.585 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0032 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.0091 uM | Not Available | BindingDB 50001885 |
References
- Fenton C, Scott LJ: Risperidone: a review of its use in the treatment of bipolar mania. CNS Drugs. 2005;19(5):429-44. [15907153 ]
- Richelson E, Souder T: Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000 Nov 24;68(1):29-39. [11132243 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Phosphatidylinositol phospholipase c activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM1
- Uniprot ID:
- P11229
- Molecular Weight:
- 51420.375 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 2.8 uM | Not Available | BindingDB 50001885 |
| Inhibitory | >10 uM | Not Available | BindingDB 50001885 |
| Inhibitory | >5 uM | Not Available | BindingDB 50001885 |
References
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Bolden C, Cusack B, Richelson E: Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992 Feb;260(2):576-80. [1346637 ]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL: Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Oct;27(7):1125-43. [14642972 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- General Function:
- Epinephrine binding
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins. The rank order of potency for agonists of this receptor is clonidine > norepinephrine > epinephrine = oxymetazoline > dopamine > p-tyramine = phenylephrine > serotonin > p-synephrine / p-octopamine. For antagonists, the rank order is yohimbine > chlorpromazine > phentolamine > mianserine > spiperone > prazosin > alprenolol > propanolol > pindolol.
- Gene Name:
- ADRA2B
- Uniprot ID:
- P18089
- Molecular Weight:
- 49565.8 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0085 uM | Not Available | BindingDB 50001885 |
References
- Fenton C, Scott LJ: Risperidone: a review of its use in the treatment of bipolar mania. CNS Drugs. 2005;19(5):429-44. [15907153 ]
- Richelson E, Souder T: Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000 Nov 24;68(1):29-39. [11132243 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Receptor signaling protein activity
- Specific Function:
- Beta-adrenergic receptors mediate the catecholamine-induced activation of adenylate cyclase through the action of G proteins. This receptor binds epinephrine and norepinephrine with approximately equal affinity. Mediates Ras activation through G(s)-alpha- and cAMP-mediated signaling.
- Gene Name:
- ADRB1
- Uniprot ID:
- P08588
- Molecular Weight:
- 51322.1 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 5 uM | Not Available | BindingDB 50001885 |
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for ergot alkaloid derivatives, various anxiolytic and antidepressant drugs and other psychoactive substances, such as lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity. Arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Regulates the release of 5-hydroxytryptamine, dopamine and acetylcholine in the brain, and thereby affects neural activity, nociceptive processing, pain perception, mood and behavior. Besides, plays a role in vasoconstriction of cerebral arteries.
- Gene Name:
- HTR1B
- Uniprot ID:
- P28222
- Molecular Weight:
- 43567.535 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0098 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.02 uM | Not Available | BindingDB 50001885 |
References
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994 May;55 Suppl:5-12. [7520908 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for ergot alkaloid derivatives, various anxiolytic and antidepressant drugs and other psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity. Regulates the release of 5-hydroxytryptamine in the brain, and thereby affects neural activity. May also play a role in regulating the release of other neurotransmitters. May play a role in vasoconstriction.
- Gene Name:
- HTR1D
- Uniprot ID:
- P28221
- Molecular Weight:
- 41906.38 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.14 uM | Not Available | BindingDB 50001885 |
References
- Richelson E, Souder T: Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000 Nov 24;68(1):29-39. [11132243 ]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various ergot alkaloid derivatives and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of dopamine and 5-hydroxytryptamine release, 5-hydroxytryptamine uptake and in the regulation of extracellular dopamine and 5-hydroxytryptamine levels, and thereby affects neural activity. May play a role in the perception of pain. Plays a role in the regulation of behavior, including impulsive behavior. Required for normal proliferation of embryonic cardiac myocytes and normal heart development. Protects cardiomyocytes against apoptosis. Plays a role in the adaptation of pulmonary arteries to chronic hypoxia. Plays a role in vasoconstriction. Required for normal osteoblast function and proliferation, and for maintaining normal bone density. Required for normal proliferation of the interstitial cells of Cajal in the intestine.
- Gene Name:
- HTR2B
- Uniprot ID:
- P41595
- Molecular Weight:
- 54297.41 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0292 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 0.115 uM | Not Available | BindingDB 50001885 |
References
- Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL: Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences. J Pharmacol Exp Ther. 1996 Feb;276(2):720-7. [8632342 ]
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO: Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res. 1999 May 4;37(1):107-22. [10227113 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that stimulate adenylate cyclase.
- Gene Name:
- HTR7
- Uniprot ID:
- P34969
- Molecular Weight:
- 53554.43 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0043 uM | Not Available | BindingDB 50001885 |
References
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Teitler M, Toohey N, Knight JA, Klein MT, Smith C: Clozapine and other competitive antagonists reactivate risperidone-inactivated h5-HT7 receptors: radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology (Berl). 2010 Dec;212(4):687-97. doi: 10.1007/s00213-010-2001-x. Epub 2010 Sep 9. [20827463 ]
- General Function:
- G-protein coupled acetylcholine receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is adenylate cyclase inhibition. Signaling promotes phospholipase C activity, leading to the release of inositol trisphosphate (IP3); this then triggers calcium ion release into the cytosol.
- Gene Name:
- CHRM2
- Uniprot ID:
- P08172
- Molecular Weight:
- 51714.605 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 3.7 uM | Not Available | BindingDB 50001885 |
| Inhibitory | >10 uM | Not Available | BindingDB 50001885 |
References
- Bolden C, Cusack B, Richelson E: Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992 Feb;260(2):576-80. [1346637 ]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL: Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Oct;27(7):1125-43. [14642972 ]
- General Function:
- Receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM3
- Uniprot ID:
- P20309
- Molecular Weight:
- 66127.445 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 50001885 |
References
- Bolden C, Cusack B, Richelson E: Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992 Feb;260(2):576-80. [1346637 ]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL: Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Oct;27(7):1125-43. [14642972 ]
- General Function:
- Guanyl-nucleotide exchange factor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is inhibition of adenylate cyclase.
- Gene Name:
- CHRM4
- Uniprot ID:
- P08173
- Molecular Weight:
- 53048.65 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 2.9 uM | Not Available | BindingDB 50001885 |
| Inhibitory | >10 uM | Not Available | BindingDB 50001885 |
References
- Bolden C, Cusack B, Richelson E: Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992 Feb;260(2):576-80. [1346637 ]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL: Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Oct;27(7):1125-43. [14642972 ]
- General Function:
- Serotonin:sodium symporter activity
- Specific Function:
- Serotonin transporter whose primary function in the central nervous system involves the regulation of serotonergic signaling via transport of serotonin molecules from the synaptic cleft back into the pre-synaptic terminal for re-utilization. Plays a key role in mediating regulation of the availability of serotonin to other receptors of serotonergic systems. Terminates the action of serotonin and recycles it in a sodium-dependent manner.
- Gene Name:
- SLC6A4
- Uniprot ID:
- P31645
- Molecular Weight:
- 70324.165 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 1 uM | Not Available | BindingDB 50001885 |
| Inhibitory | 1.4 uM | Not Available | BindingDB 50001885 |
References
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various alkaloids and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity.
- Gene Name:
- HTR1E
- Uniprot ID:
- P28566
- Molecular Weight:
- 41681.57 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 1.32 uM | Not Available | BindingDB 50001885 |
References
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various alkaloids and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity.
- Gene Name:
- HTR1F
- Uniprot ID:
- P30939
- Molecular Weight:
- 41708.505 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 1.24 uM | Not Available | BindingDB 50001885 |
References
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. [8935801 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- This alpha-adrenergic receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Its effect is mediated by G(q) and G(11) proteins. Nuclear ADRA1A-ADRA1B heterooligomers regulate phenylephrine (PE)-stimulated ERK signaling in cardiac myocytes.
- Gene Name:
- ADRA1B
- Uniprot ID:
- P35368
- Molecular Weight:
- 56835.375 Da
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which activate adenylyl cyclase.
- Gene Name:
- DRD5
- Uniprot ID:
- P21918
- Molecular Weight:
- 52950.5 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.563 uM | Not Available | BindingDB 50001885 |
References
- Kongsamut S, Roehr JE, Cai J, Hartman HB, Weissensee P, Kerman LL, Tang L, Sandrasagra A: Iloperidone binding to human and rat dopamine and 5-HT receptors. Eur J Pharmacol. 1996 Dec 19;317(2-3):417-23. [8997630 ]
- General Function:
- Monovalent cation:proton antiporter activity
- Specific Function:
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide (NMN), metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfate, acyclovir, ganciclovir and also the zwitterionic cephalosporin, cephalexin and cephradin. Seems to also play a role in the uptake of oxaliplatin (a new platinum anticancer agent). Able to transport paraquat (PQ or N,N-dimethyl-4-4'-bipiridinium); a widely used herbicid. Responsible for the secretion of cationic drugs across the brush border membranes.
- Gene Name:
- SLC47A1
- Uniprot ID:
- Q96FL8
- Molecular Weight:
- 61921.585 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 1.6 uM | Not Available | BindingDB 50001885 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- General Function:
- Drug transmembrane transporter activity
- Specific Function:
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide, metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfate, acyclovir, and ganciclovir. Responsible for the secretion of cationic drugs across the brush border membranes.
- Gene Name:
- SLC47A2
- Uniprot ID:
- Q86VL8
- Molecular Weight:
- 65083.915 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 291 uM | Not Available | BindingDB 50001885 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- General Function:
- Opioid receptor activity
- Specific Function:
- Functions in lipid transport from the endoplasmic reticulum and is involved in a wide array of cellular functions probably through regulation of the biogenesis of lipid microdomains at the plasma membrane. Involved in the regulation of different receptors it plays a role in BDNF signaling and EGF signaling. Also regulates ion channels like the potassium channel and could modulate neurotransmitter release. Plays a role in calcium signaling through modulation together with ANK2 of the ITP3R-dependent calcium efflux at the endoplasmic reticulum. Plays a role in several other cell functions including proliferation, survival and death. Originally identified for its ability to bind various psychoactive drugs it is involved in learning processes, memory and mood alteration (PubMed:16472803, PubMed:9341151). Necessary for proper mitochondrial axonal transport in motor neurons, in particular the retrograde movement of mitochondria (By similarity).
- Gene Name:
- SIGMAR1
- Uniprot ID:
- Q99720
- Molecular Weight:
- 25127.52 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 4.3 uM | Not Available | BindingDB 50001885 |
References
- Bolos J, Anglada L, Gubert S, Planas JM, Agut J, Princep M, De la Fuente N, Sacristan A, Ortiz JA: 7-[3-(1-piperidinyl)propoxy]chromenones as potential atypical antipsychotics. 2. Pharmacological profile of 7-[3-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)-piperidin-1-yl]propoxy]-3-(hydroxymeth yl)chromen -4-one (abaperidone, FI-8602). J Med Chem. 1998 Dec 31;41(27):5402-9. [9876110 ]
- General Function:
- Quaternary ammonium group transmembrane transporter activity
- Specific Function:
- Mediates tubular uptake of organic compounds from circulation. Mediates the influx of agmatine, dopamine, noradrenaline (norepinephrine), serotonin, choline, famotidine, ranitidine, histamin, creatinine, amantadine, memantine, acriflavine, 4-[4-(dimethylamino)-styryl]-N-methylpyridinium ASP, amiloride, metformin, N-1-methylnicotinamide (NMN), tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, cisplatin and oxaliplatin. Cisplatin may develop a nephrotoxic action. Transport of creatinine is inhibited by fluoroquinolones such as DX-619 and LVFX. This transporter is a major determinant of the anticancer activity of oxaliplatin and may contribute to antitumor specificity.
- Gene Name:
- SLC22A2
- Uniprot ID:
- O15244
- Molecular Weight:
- 62579.99 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 11 uM | Not Available | BindingDB 50001885 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]