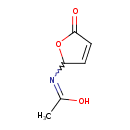
Butenolide (T3D3753)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2010-05-13 14:52:29 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:26:29 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D3753 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Butenolide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Butenolide is a mycotoxin found in various species of fungi of the genus Fusarium. It can often be found in contaminated agricultural products. Butenolide had been implicated as the causative agent of a livestock mycotoxicosis called “fescue foot”, a peripheral vascular disorder occurring in cattle grazing on tall fescue grass. Butenolide is also one of the mycotoxins which have been considered as a suspected etiological factor for Kaschin-Beck disease (an endemic osteoarthropathy) and Keshan disease (an endemic cardiomyopathy), prevailing in some regions of China, and several studies have also indicated that butenolide can cause cartilage damage. (1, 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C6H7NO3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 141.125 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 141.043 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 16275-44-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | N-(5-oxo-2,5-dihydrofuran-2-yl)ethanimidic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | N-(5-oxo-2H-furan-2-yl)ethanimidic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CC(O)=NC1OC(=O)C=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1/C6H7NO3/c1-4(8)7-5-2-3-6(9)10-5/h2-3,5H,1H3,(H,7,8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=HUSDLVGPEKVWAL-UHFFFAOYNA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as butenolides. These are dihydrofurans with a carbonyl group at the C2 carbon atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Dihydrofurans | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Furanones | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Butenolides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral, dermal, inhalation, and parenteral (contaminated drugs). (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Butenolide causes lipid peroxidation, disrupting the membrane lipid bilayer and causing damage to membrane protiens. It also induces production of reactive oxygen species by impairing the activities of complexes I–IV of the mitochondrial respiratory chain, especially in the liver, leading to oxidative stress. In addition, butenolide disrupts the cation gradient by inhibiting the activity of Ca2+/Mg2+-ATPase and Na+/K+-ATPase, likely either as a side effect of lipid peroxidation or by binding to the sulfhydryl groups in the active sites of these enzymes. (1, 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 44 mg/kg (Intraperitoneal, Mouse) (3) LD50: 275 mg/kg (Oral, Mouse) (3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | Not listed by IARC. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Butenolide is a mycotoxin found in various species of fungi of the genus Fusarium. It can often be found in contaminated agricultural products. (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Butenolide is cardiotoxic and hepatotoxic. Fusarium mycotoxins has been associated with both alimentary toxic aleukia, and esophageal cancer. Consumption of Fusarium mycotoxins-contaminated foodstuffs has also been considered as a suspected etiological factor for Kashin–Beck disease and Keshan disease. (1, 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Butenolide irritates the eyes and skin. It may also cause weight loss and growth retardation. (1, 3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 27790 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Butenolide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D3753.pdf | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Signal transducer activity
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of the calcium.
- Gene Name:
- ATP2C1
- Uniprot ID:
- P98194
- Molecular Weight:
- 100576.42 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium.
- Gene Name:
- ATP2C2
- Uniprot ID:
- O75185
- Molecular Weight:
- 103186.475 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Pdz domain binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B1
- Uniprot ID:
- P20020
- Molecular Weight:
- 138754.045 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Protein c-terminus binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B2
- Uniprot ID:
- Q01814
- Molecular Weight:
- 136875.18 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Pdz domain binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B3
- Uniprot ID:
- Q16720
- Molecular Weight:
- 134196.025 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Scaffold protein binding
- Specific Function:
- Calcium/calmodulin-regulated and magnesium-dependent enzyme that catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell (PubMed:8530416). By regulating sperm cell calcium homeostasis, may play a role in sperm motility (By similarity).
- Gene Name:
- ATP2B4
- Uniprot ID:
- P23634
- Molecular Weight:
- 137919.03 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Key regulator of striated muscle performance by acting as the major Ca(2+) ATPase responsible for the reuptake of cytosolic Ca(2+) into the sarcoplasmic reticulum. Catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A1
- Uniprot ID:
- O14983
- Molecular Weight:
- 110251.36 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- S100 protein binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Isoform 2 is involved in the regulation of the contraction/relaxation cycle.
- Gene Name:
- ATP2A2
- Uniprot ID:
- P16615
- Molecular Weight:
- 114755.765 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium. Transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A3
- Uniprot ID:
- Q93084
- Molecular Weight:
- 113976.23 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A1
- Uniprot ID:
- P05023
- Molecular Weight:
- 112895.01 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A2
- Uniprot ID:
- P50993
- Molecular Weight:
- 112264.385 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A3
- Uniprot ID:
- P13637
- Molecular Weight:
- 111747.51 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients. Plays a role in sperm motility.
- Gene Name:
- ATP1A4
- Uniprot ID:
- Q13733
- Molecular Weight:
- 114165.44 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The beta subunit regulates, through assembly of alpha/beta heterodimers, the number of sodium pumps transported to the plasma membrane.Involved in cell adhesion and establishing epithelial cell polarity.
- Gene Name:
- ATP1B1
- Uniprot ID:
- P05026
- Molecular Weight:
- 35061.07 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The exact function of the beta-2 subunit is not known.Mediates cell adhesion of neurons and astrocytes, and promotes neurite outgrowth.
- Gene Name:
- ATP1B2
- Uniprot ID:
- P14415
- Molecular Weight:
- 33366.925 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The exact function of the beta-3 subunit is not known.
- Gene Name:
- ATP1B3
- Uniprot ID:
- P54709
- Molecular Weight:
- 31512.34 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]
- General Function:
- Transporter activity
- Specific Function:
- May be involved in forming the receptor site for cardiac glycoside binding or may modulate the transport function of the sodium ATPase.
- Gene Name:
- FXYD2
- Uniprot ID:
- P54710
- Molecular Weight:
- 7283.265 Da
References
- Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY: The oxidative damage of butenolide to isolated erythrocyte membranes. Toxicol In Vitro. 2007 Aug;21(5):863-9. Epub 2007 Feb 28. [17416482 ]