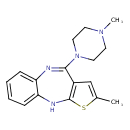
Olanzapine (T3D2754)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:26:38 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:51 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2754 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Olanzapine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Olanzapine was the third atypical antipsychotic to gain approval by the Food and Drug Administration (FDA) and has become one of the most commonly used atypical antipsychotics. Olanzapine has been approved by the FDA for the treatment of schizophrenia, acute mania in bipolar disorder, agitation associated with schizophrenia and bipolar disorder, and as maintenance treatment in bipolar disorder and psychotic depression. It has also been established in treating depression off-label because of its mood-stabilizing properties and its ability to increase the efficacy of antidepressants. Olanzapine is manufactured and marketed by the pharmaceutical company Eli Lilly and Company. It is available as a pill that comes in the strengths of 2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, and 20 mg and as as Zydis orally disintegrating tablets in the strengths of 5 mg, 10 mg, 15 mg, and 20 mg. It is also available as a rapid-acting intramuscular injection for short term acute use. Olanzapine (oh-LAN-za-peen, sold as Zyprexa, Zydis, or in combination with fluoxetine, as Symbyax) was the third atypical antipsychotic to gain approval by the Food and Drug Administration (FDA) and has become one of the most commonly used atypical antipsychotics. Olanzapine has been approved by the FDA for the treatment of schizophrenia, acute mania in bipolar disorder, agitation associated with schizophrenia and bipolar disorder, and as maintenance treatment in bipolar disorder and psychotic depression. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C17H20N4S | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 312.433 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 312.141 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 132539-06-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 5-methyl-8-(4-methylpiperazin-1-yl)-4-thia-2,9-diazatricyclo[8.4.0.0³,⁷]tetradeca-1(14),3(7),5,8,10,12-hexaene | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | zyprexa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CN1CCN(CC1)C1=NC2=CC=CC=C2NC2=C1C=C(C)S2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=KVWDHTXUZHCGIO-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as benzodiazepines. These are organic compounds containing a benzene ring fused to either isomers of diazepine(unsaturated seven-member heterocycle with two nitrogen atoms replacing two carbon atoms). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Intramuscular, Oral. Well absorbed, with approximately 40% of the dose metabolized before reaching the systemic circulation. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Olanzapine's antipsychotic activity is likely due to a combination of antagonism at D2 receptors in the mesolimbic pathway and 5HT2A receptors in the frontal cortex. Antagonism at D2 receptors relieves positive symptoms while antagonism at 5HT2A receptors relieves negative symptoms of schizophrenia. Olanzapine is an antagonist at types 1, 2, and 4 dopamine receptors, 5-HT receptor types 2A and 2C, muscarinic receptors 1 through 5, alpha(1)-receptors, and histamine H1-receptors. Olanzapine's antipsychotic effect is due to antagonism at dopamine and serotonin type 2 receptors, with greater activity at serotonin 5-HT2 receptors than at dopamine type-2 receptors. This may explain the lack of extrapyramidal effects. Olanzapine does not appear to block dopamine within the tubero-infundibular tract, explaining the lower incidence of hyperprolactinemia than with typical antipsychotic agents or risperidone. Antagonism at muscarinic receptors, H1-receptors, and alpha(1)-receptors also occurs with olanzapine. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Hepatic Route of Elimination: It is eliminated extensively by first pass metabolism, with approximately 40% of the dose metabolized before reaching the systemic circulation. Following a single oral dose of 14C labeled olanzapine, 7% of the dose of olanzapine was recovered in the urine as unchanged drug, indicating that olanzapine is highly metabolized. Half Life: 21 to 54 hours | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Death has been reported after an acute overdose of 0.45 g, but also survival after an acute overdose of 1500 g. (6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | Not listed by IARC. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For the acute and maintenance treatment of schizophrenia and related psychotic disorders, as well as acute treatment of manic or mixed episodes of bipolar 1 disorder. Intramuscular olanzapine is indicated for the rapid control of agitated patients. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | The principal side effect of olanzapine is weight gain, which may be profound in some cases and/or associated with derangement in the blood lipid and blood sugar profiles. Extrapyramidal side effects, although potentially serious, are infrequent to rare from olanzapine but may include tremors and muscle rigidity. Several patient groups are at a heightened risk of side effects from olanzapine and antipsychotics in general. Olanzapine may produce non-trivial hyperglycemia in patients with diabetes mellitus. Likewise, the elderly are at a greater risk of falls and accidental injury. Young males appear to be at heightened risk of dystonic reactions, although these are relatively rare with olanzapine. Most antipsychotics, including olanzapine, may disrupt the body's natural thermoregulatory systems, thus permitting excursions to dangerous levels when situations (exposure to heat, strenuous exercise) occur. While olanzapine is used therapeutically to treat serious mental illness, occasionally it can have the opposite effect and provoke serious paradoxical reactions in a small subgroup of people, with the drug causing unusual changes in personality, thoughts or behavior; hallucinations and suicidal ideation have also been linked to olanzapine use. The U.S. Food and Drug Administration requires all atypical antipsychotics to include a warning about the risk of developing hyperglycemia and diabetes, both of which are factors in the metabolic syndrome. These effects may be related to the drugs' ability to induce weight gain, although there are some reports of metabolic changes in the absence of weight gain. Olanzapine has demonstrated carcinogenic effects in multiple studies when exposed chronically to female mice and rats, but not male mice and rats. The tumors found were in either the liver or mammary glands of the animals. Symptoms of an overdose include tachycardia, agitation, dysarthria, decreased consciousness and coma. Death may result from acute overdose. (Wikipedia) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of an overdose include tachycardia, agitation, dysarthria, decreased consciousness and coma. Death has been reported after an acute overdose of 0.45g, but also survival after an acute overdose of 1500g. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | In case of acute overdose, establish and maintain an airway and ensure adequate ventilation, which may include intubation. Induction of emesis is not recommended as the possibility of obtundation, seizures, or dystonic reactions of the head and neck following overdose may create a risk for aspiration. Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids and/or sympathomimetic agents. (13) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00334 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB05012 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 4585 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL715 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 10442212 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C07322 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 7735 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Olanzapine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Olanzapine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Charles Arthur Bunnell, Samuel Dean Larsen, John Richard Nichols, Susan Marie Reutzel, Gregory Alan Stephenson, “Intermediates and process for preparing olanzapine.” U.S. Patent US6020487, issued September, 1996. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Virus receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including mescaline, psilocybin, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates phospholipase C and a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and promotes the release of Ca(2+) ions from intracellular stores. Affects neural activity, perception, cognition and mood. Plays a role in the regulation of behavior, including responses to anxiogenic situations and psychoactive substances. Plays a role in intestinal smooth muscle contraction, and may play a role in arterial vasoconstriction.(Microbial infection) Acts as a receptor for human JC polyomavirus/JCPyV.
- Gene Name:
- HTR2A
- Uniprot ID:
- P28223
- Molecular Weight:
- 52602.58 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0019 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0023 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0033 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.004 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.007 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.009 uM | Not Available | BindingDB 50053711 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- McDonald LM, Moran PM, Vythelingum GN, Joseph MH, Stephenson JD, Gray JA: Enhancement of latent inhibition by two 5-HT2A receptor antagonists only when given at both pre-exposure and conditioning. Psychopharmacology (Berl). 2003 Sep;169(3-4):321-31. Epub 2002 Aug 9. [14530903 ]
- Moresco RM, Cavallaro R, Messa C, Bravi D, Gobbo C, Galli L, Lucignani G, Colombo C, Rizzo G, Velona I, Smeraldi E, Fazio F: Cerebral D2 and 5-HT2 receptor occupancy in Schizophrenic patients treated with olanzapine or clozapine. J Psychopharmacol. 2004 Sep;18(3):355-65. [15358979 ]
- Sharpley AL, Attenburrow ME, Hafizi S, Cowen PJ: Olanzapine increases slow wave sleep and sleep continuity in SSRI-resistant depressed patients. J Clin Psychiatry. 2005 Apr;66(4):450-4. [15816787 ]
- Yatham LN, Goldstein JM, Vieta E, Bowden CL, Grunze H, Post RM, Suppes T, Calabrese JR: Atypical antipsychotics in bipolar depression: potential mechanisms of action. J Clin Psychiatry. 2005;66 Suppl 5:40-8. [16038601 ]
- Padin JF, Rodriguez MA, Dominguez E, Dopeso-Reyes IG, Buceta M, Cano E, Sotelo E, Brea J, Caruncho HJ, Isabel Cadavid M, Castro M, Isabel Loza M: Parallel regulation by olanzapine of the patterns of expression of 5-HT2A and D3 receptors in rat central nervous system and blood cells. Neuropharmacology. 2006 Sep;51(4):923-32. Epub 2006 Aug 14. [16905159 ]
- Gao M, Shi Z, Wang M, Zheng QH: [11C]olanzapine, radiosynthesis and lipophilicity of a new potential PET 5-HT2 and D2 receptor radioligand. Bioorg Med Chem Lett. 2013 Apr 1;23(7):1953-6. doi: 10.1016/j.bmcl.2013.02.045. Epub 2013 Feb 14. [23466228 ]
- Hrib NJ, Jurcak JG, Bregna DE, Burgher KL, Hartman HB, Kafka S, Kerman LL, Kongsamut S, Roehr JE, Szewczak MR, Woods-Kettelberger AT, Corbett R: Structure-activity relationships of a series of novel (piperazinylbutyl)thiazolidinone antipsychotic agents related to 3-[4-[4-(6-fluorobenzo[b]thien-3-yl)-1-piperazinyl]butyl]-2,5,5- trimethyl-4-thiazolidinone maleate. J Med Chem. 1996 Sep 27;39(20):4044-57. [8831770 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Heffernan GD, Coghlan RD, Manas ES, McDevitt RE, Li Y, Mahaney PE, Robichaud AJ, Huselton C, Alfinito P, Bray JA, Cosmi SA, Johnston GH, Kenney T, Koury E, Winneker RC, Deecher DC, Trybulski EJ: Dual acting norepinephrine reuptake inhibitors and 5-HT(2A) receptor antagonists: Identification, synthesis and activity of novel 4-aminoethyl-3-(phenylsulfonyl)-1H-indoles. Bioorg Med Chem. 2009 Nov 15;17(22):7802-15. doi: 10.1016/j.bmc.2009.09.023. Epub 2009 Sep 18. [19836247 ]
- Melkersson KI, Gunes A, Dahl ML: Impact of serotonin receptor 2A gene haplotypes on C-peptide levels in clozapine- and olanzapine-treated patients. Hum Psychopharmacol. 2010 Jun-Jul;25(4):347-52. doi: 10.1002/hup.1114. [20521326 ]
- General Function:
- Potassium channel regulator activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which inhibit adenylyl cyclase.
- Gene Name:
- DRD2
- Uniprot ID:
- P14416
- Molecular Weight:
- 50618.91 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0021 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0061 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.011 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.017 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.02 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.032 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.078 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.01 uM | Not Available | BindingDB 50053711 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Naiker DV, Catts SV, Catts VS, Bedi KS, Bryan-Lluka LJ: Dose determination of haloperidol, risperidone and olanzapine using an in vivo dopamine D2-receptor occupancy method in the rat. Eur J Pharmacol. 2006 Jul 1;540(1-3):87-90. Epub 2006 May 11. [16730699 ]
- Weizman T, Pick CG, Backer MM, Rigai T, Bloch M, Schreiber S: The antinociceptive effect of amisulpride in mice is mediated through opioid mechanisms. Eur J Pharmacol. 2003 Oct 8;478(2-3):155-9. [14575800 ]
- Jordan S, Regardie K, Johnson JL, Chen R, Kambayashi J, McQuade R, Kitagawa H, Tadori Y, Kikuchi T: In vitro functional characteristics of dopamine D2 receptor partial agonists in second and third messenger-based assays of cloned human dopamine D2Long receptor signalling. J Psychopharmacol. 2007 Aug;21(6):620-7. Epub 2006 Nov 8. [17092971 ]
- Thacker SK, Perna MK, Ward JJ, Schaefer TL, Williams MT, Kostrzewa RM, Brown RW: The effects of adulthood olanzapine treatment on cognitive performance and neurotrophic factor content in male and female rats neonatally treated with quinpirole. Eur J Neurosci. 2006 Oct;24(7):2075-83. [17067304 ]
- Lencz T, Robinson DG, Xu K, Ekholm J, Sevy S, Gunduz-Bruce H, Woerner MG, Kane JM, Goldman D, Malhotra AK: DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am J Psychiatry. 2006 Mar;163(3):529-31. [16513877 ]
- Gao M, Shi Z, Wang M, Zheng QH: [11C]olanzapine, radiosynthesis and lipophilicity of a new potential PET 5-HT2 and D2 receptor radioligand. Bioorg Med Chem Lett. 2013 Apr 1;23(7):1953-6. doi: 10.1016/j.bmcl.2013.02.045. Epub 2013 Feb 14. [23466228 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Pettersson F, Ponten H, Waters N, Waters S, Sonesson C: Synthesis and evaluation of a set of 4-phenylpiperidines and 4-phenylpiperazines as D2 receptor ligands and the discovery of the dopaminergic stabilizer 4-[3-(methylsulfonyl)phenyl]-1-propylpiperidine (huntexil, pridopidine, ACR16). J Med Chem. 2010 Mar 25;53(6):2510-20. doi: 10.1021/jm901689v. [20155917 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- de Haan L, Lavalaye J, Linszen D, Dingemans PM, Booij J: Subjective experience and striatal dopamine D(2) receptor occupancy in patients with schizophrenia stabilized by olanzapine or risperidone. Am J Psychiatry. 2000 Jun;157(6):1019-20. [10831489 ]
- Arakawa R, Okumura M, Ito H, Takano A, Takahashi H, Takano H, Maeda J, Okubo Y, Suhara T: Positron emission tomography measurement of dopamine D(2) receptor occupancy in the pituitary and cerebral cortex: relation to antipsychotic-induced hyperprolactinemia. J Clin Psychiatry. 2010 Sep;71(9):1131-7. doi: 10.4088/JCP.08m04307yel. Epub 2010 Feb 23. [20361897 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances, including ergot alkaloid derivatives, 1-2,5,-dimethoxy-4-iodophenyl-2-aminopropane (DOI) and lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Regulates neuronal activity via the activation of short transient receptor potential calcium channels in the brain, and thereby modulates the activation of pro-opiomelacortin neurons and the release of CRH that then regulates the release of corticosterone. Plays a role in the regulation of appetite and eating behavior, responses to anxiogenic stimuli and stress. Plays a role in insulin sensitivity and glucose homeostasis.
- Gene Name:
- HTR2C
- Uniprot ID:
- P28335
- Molecular Weight:
- 51820.705 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0028 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0071 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.01 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.011 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.014 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.071 uM | Not Available | BindingDB 50053711 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Wood MD, Scott C, Clarke K, Cato KJ, Patel N, Heath J, Worby A, Gordon L, Campbell L, Riley G, Davies CH, Gribble A, Jones DN: Pharmacological profile of antipsychotics at monoamine receptors: atypicality beyond 5-HT2A receptor blockade. CNS Neurol Disord Drug Targets. 2006 Aug;5(4):445-52. [16918396 ]
- Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J: Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms. Biochem Pharmacol. 2006 Feb 14;71(4):521-9. Epub 2005 Dec 5. [16336943 ]
- Overstreet DH, Knapp DJ, Breese GR: Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin Exp Res. 2007 Sep;31(9):1473-81. Epub 2007 Jul 11. [17624999 ]
- Theisen FM, Haberhausen M, Firnges MA, Gregory P, Reinders JH, Remschmidt H, Hebebrand J, Antel J: No evidence for binding of clozapine, olanzapine and/or haloperidol to selected receptors involved in body weight regulation. Pharmacogenomics J. 2007 Aug;7(4):275-81. Epub 2006 Sep 19. [16983399 ]
- Hertel P: Comparing sertindole to other new generation antipsychotics on preferential dopamine output in limbic versus striatal projection regions: mechanism of action. Synapse. 2006 Dec 1;60(7):543-52. [16952163 ]
- Gao M, Shi Z, Wang M, Zheng QH: [11C]olanzapine, radiosynthesis and lipophilicity of a new potential PET 5-HT2 and D2 receptor radioligand. Bioorg Med Chem Lett. 2013 Apr 1;23(7):1953-6. doi: 10.1016/j.bmcl.2013.02.045. Epub 2013 Feb 14. [23466228 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Krogsgaard-Larsen N, Jensen AA, Kehler J: Novel 7-phenylsulfanyl-1,2,3,4,10,10a-hexahydro-pyrazino[1,2-a]indoles as dual serotonin 5-HT2C and 5-HT6 receptor ligands. Bioorg Med Chem Lett. 2010 Sep 15;20(18):5431-3. doi: 10.1016/j.bmcl.2010.07.105. Epub 2010 Aug 3. [20719507 ]
- General Function:
- Histamine receptor activity
- Specific Function:
- In peripheral tissues, the H1 subclass of histamine receptors mediates the contraction of smooth muscles, increase in capillary permeability due to contraction of terminal venules, and catecholamine release from adrenal medulla, as well as mediating neurotransmission in the central nervous system.
- Gene Name:
- HRH1
- Uniprot ID:
- P35367
- Molecular Weight:
- 55783.61 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0012 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0028 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0035 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0056 uM | Not Available | BindingDB 50053711 |
| Dissociation | 0.000087 uM | Not Available | BindingDB 50053711 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Poyurovsky M, Pashinian A, Levi A, Weizman R, Weizman A: The effect of betahistine, a histamine H1 receptor agonist/H3 antagonist, on olanzapine-induced weight gain in first-episode schizophrenia patients. Int Clin Psychopharmacol. 2005 Mar;20(2):101-3. [15729086 ]
- Rasmussen K, Benvenga MJ, Bymaster FP, Calligaro DO, Cohen IR, Falcone JF, Hemrick-Luecke SK, Martin FM, Moore NA, Nisenbaum LK, Schaus JM, Sundquist SJ, Tupper DE, Wiernicki TR, Nelson DL: Preclinical pharmacology of FMPD [6-fluoro-10-[3-(2-methoxyethyl)-4-methyl-piperazin-1-yl]-2-methyl-4H-3-thia-4,9- diaza-benzo[f]azulene]: a potential novel antipsychotic with lower histamine H1 receptor affinity than olanzapine. J Pharmacol Exp Ther. 2005 Dec;315(3):1265-77. Epub 2005 Sep 1. [16141369 ]
- Altschuler EL, Kast RE: Using histamine (H1) antagonists, in particular atypical antipsychotics, to treat anemia of chronic disease via interleukin-6 suppression. Med Hypotheses. 2005;65(1):65-7. [15893120 ]
- Liu T, Lin Y, Wen X, Jorissen RN, Gilson MK: BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007 Jan;35(Database issue):D198-201. Epub 2006 Dec 1. [17145705 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Theisen FM, Haberhausen M, Firnges MA, Gregory P, Reinders JH, Remschmidt H, Hebebrand J, Antel J: No evidence for binding of clozapine, olanzapine and/or haloperidol to selected receptors involved in body weight regulation. Pharmacogenomics J. 2007 Aug;7(4):275-81. Epub 2006 Sep 19. [16983399 ]
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which inhibit adenylyl cyclase. Promotes cell proliferation.
- Gene Name:
- DRD3
- Uniprot ID:
- P35462
- Molecular Weight:
- 44224.335 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.039 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.043 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.045 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.049 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.054 uM | Not Available | BindingDB 50053711 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Campiani G, Butini S, Fattorusso C, Trotta F, Gemma S, Catalanotti B, Nacci V, Fiorini I, Cagnotto A, Mereghetti I, Mennini T, Minetti P, Di Cesare MA, Stasi MA, Di Serio S, Ghirardi O, Tinti O, Carminati P: Novel atypical antipsychotic agents: rational design, an efficient palladium-catalyzed route, and pharmacological studies. J Med Chem. 2005 Mar 24;48(6):1705-8. [15771414 ]
- Butini S, Gemma S, Campiani G, Franceschini S, Trotta F, Borriello M, Ceres N, Ros S, Coccone SS, Bernetti M, De Angelis M, Brindisi M, Nacci V, Fiorini I, Novellino E, Cagnotto A, Mennini T, Sandager-Nielsen K, Andreasen JT, Scheel-Kruger J, Mikkelsen JD, Fattorusso C: Discovery of a new class of potential multifunctional atypical antipsychotic agents targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors: design, synthesis, and effects on behavior. J Med Chem. 2009 Jan 8;52(1):151-69. doi: 10.1021/jm800689g. [19072656 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- General Function:
- Sh3 domain binding
- Specific Function:
- Dopamine receptor responsible for neuronal signaling in the mesolimbic system of the brain, an area of the brain that regulates emotion and complex behavior. Its activity is mediated by G proteins which inhibit adenylyl cyclase. Modulates the circadian rhythm of contrast sensitivity by regulating the rhythmic expression of NPAS2 in the retinal ganglion cells (By similarity).
- Gene Name:
- DRD4
- Uniprot ID:
- P21917
- Molecular Weight:
- 48359.86 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.028 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.029 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.05 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.173 uM | Not Available | BindingDB 50053711 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, Swift R: The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006 Jun;31(6):1310-7. [16237394 ]
- Hrib NJ, Jurcak JG, Bregna DE, Burgher KL, Hartman HB, Kafka S, Kerman LL, Kongsamut S, Roehr JE, Szewczak MR, Woods-Kettelberger AT, Corbett R: Structure-activity relationships of a series of novel (piperazinylbutyl)thiazolidinone antipsychotic agents related to 3-[4-[4-(6-fluorobenzo[b]thien-3-yl)-1-piperazinyl]butyl]-2,5,5- trimethyl-4-thiazolidinone maleate. J Med Chem. 1996 Sep 27;39(20):4044-57. [8831770 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- General Function:
- Voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization
- Specific Function:
- Pore-forming (alpha) subunit of voltage-gated inwardly rectifying potassium channel. Channel properties are modulated by cAMP and subunit assembly. Mediates the rapidly activating component of the delayed rectifying potassium current in heart (IKr). Isoforms USO have no channel activity by themself, but modulates channel characteristics by forming heterotetramers with other isoforms which are retained intracellularly and undergo ubiquitin-dependent degradation.
- Gene Name:
- KCNH2
- Uniprot ID:
- Q12809
- Molecular Weight:
- 126653.52 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 36 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.181 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.18197 uM | Not Available | BindingDB 50053711 |
| IC50 | 0.182 uM | Not Available | BindingDB 50053711 |
References
- Thai KM, Ecker GF: A binary QSAR model for classification of hERG potassium channel blockers. Bioorg Med Chem. 2008 Apr 1;16(7):4107-19. doi: 10.1016/j.bmc.2008.01.017. Epub 2008 Jan 16. [18243713 ]
- Tobita M, Nishikawa T, Nagashima R: A discriminant model constructed by the support vector machine method for HERG potassium channel inhibitors. Bioorg Med Chem Lett. 2005 Jun 2;15(11):2886-90. [15911273 ]
- Jia L, Sun H: Support vector machines classification of hERG liabilities based on atom types. Bioorg Med Chem. 2008 Jun 1;16(11):6252-60. doi: 10.1016/j.bmc.2008.04.028. Epub 2008 Apr 16. [18448342 ]
- Keseru GM: Prediction of hERG potassium channel affinity by traditional and hologram qSAR methods. Bioorg Med Chem Lett. 2003 Aug 18;13(16):2773-5. [12873512 ]
- Butini S, Gemma S, Campiani G, Franceschini S, Trotta F, Borriello M, Ceres N, Ros S, Coccone SS, Bernetti M, De Angelis M, Brindisi M, Nacci V, Fiorini I, Novellino E, Cagnotto A, Mennini T, Sandager-Nielsen K, Andreasen JT, Scheel-Kruger J, Mikkelsen JD, Fattorusso C: Discovery of a new class of potential multifunctional atypical antipsychotic agents targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors: design, synthesis, and effects on behavior. J Med Chem. 2009 Jan 8;52(1):151-69. doi: 10.1021/jm800689g. [19072656 ]
- Butini S, Campiani G, Franceschini S, Trotta F, Kumar V, Guarino E, Borrelli G, Fiorini I, Novellino E, Fattorusso C, Persico M, Orteca N, Sandager-Nielsen K, Jacobsen TA, Madsen K, Scheel-Kruger J, Gemma S: Discovery of bishomo(hetero)arylpiperazines as novel multifunctional ligands targeting dopamine D(3) and serotonin 5-HT(1A) and 5-HT(2A) receptors. J Med Chem. 2010 Jun 24;53(12):4803-7. doi: 10.1021/jm100294b. [20481570 ]
- General Function:
- Phosphatidylinositol phospholipase c activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM1
- Uniprot ID:
- P11229
- Molecular Weight:
- 51420.375 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0021 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.0047 uM | Not Available | BindingDB 50053711 |
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Theisen FM, Haberhausen M, Firnges MA, Gregory P, Reinders JH, Remschmidt H, Hebebrand J, Antel J: No evidence for binding of clozapine, olanzapine and/or haloperidol to selected receptors involved in body weight regulation. Pharmacogenomics J. 2007 Aug;7(4):275-81. Epub 2006 Sep 19. [16983399 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that stimulate adenylate cyclase. It has a high affinity for tricyclic psychotropic drugs (By similarity). Controls pyramidal neurons migration during corticogenesis, through the regulation of CDK5 activity (By similarity). Is an activator of TOR signaling (PubMed:23027611).
- Gene Name:
- HTR6
- Uniprot ID:
- P50406
- Molecular Weight:
- 46953.625 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.006 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.01 uM | Not Available | BindingDB 50053711 |
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- Krogsgaard-Larsen N, Jensen AA, Kehler J: Novel 7-phenylsulfanyl-1,2,3,4,10,10a-hexahydro-pyrazino[1,2-a]indoles as dual serotonin 5-HT2C and 5-HT6 receptor ligands. Bioorg Med Chem Lett. 2010 Sep 15;20(18):5431-3. doi: 10.1016/j.bmcl.2010.07.105. Epub 2010 Aug 3. [20719507 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Theisen FM, Haberhausen M, Firnges MA, Gregory P, Reinders JH, Remschmidt H, Hebebrand J, Antel J: No evidence for binding of clozapine, olanzapine and/or haloperidol to selected receptors involved in body weight regulation. Pharmacogenomics J. 2007 Aug;7(4):275-81. Epub 2006 Sep 19. [16983399 ]
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which activate adenylyl cyclase.
- Gene Name:
- DRD1
- Uniprot ID:
- P21728
- Molecular Weight:
- 49292.765 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.01 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.052 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 0.25 uM | Not Available | BindingDB 50053711 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Wermuth CG: Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303-14. [14998318 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Gene Name:
- HTR1A
- Uniprot ID:
- P08908
- Molecular Weight:
- 46106.335 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 2.1 uM | Not Available | BindingDB 50053711 |
| Inhibitory | 7.1 uM | Not Available | BindingDB 50053711 |
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]
- Rowley M, Bristow LJ, Hutson PH: Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001 Feb 15;44(4):477-501. [11170639 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that stimulate adenylate cyclase.
- Gene Name:
- HTR7
- Uniprot ID:
- P34969
- Molecular Weight:
- 53554.43 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.365 uM | Not Available | BindingDB 50053711 |
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- This alpha-adrenergic receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Its effect is mediated by G(q) and G(11) proteins. Nuclear ADRA1A-ADRA1B heterooligomers regulate phenylephrine(PE)-stimulated ERK signaling in cardiac myocytes.
- Gene Name:
- ADRA1A
- Uniprot ID:
- P35348
- Molecular Weight:
- 51486.005 Da
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO: Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res. 1999 May 4;37(1):107-22. [10227113 ]
- General Function:
- Thioesterase binding
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins. The rank order of potency for agonists of this receptor is oxymetazoline > clonidine > epinephrine > norepinephrine > phenylephrine > dopamine > p-synephrine > p-tyramine > serotonin = p-octopamine. For antagonists, the rank order is yohimbine > phentolamine = mianserine > chlorpromazine = spiperone = prazosin > propanolol > alprenolol = pindolol.
- Gene Name:
- ADRA2A
- Uniprot ID:
- P08913
- Molecular Weight:
- 48956.275 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.192 uM | Not Available | BindingDB 50053711 |
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins.
- Gene Name:
- ADRA2C
- Uniprot ID:
- P18825
- Molecular Weight:
- 49521.585 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.082 uM | Not Available | BindingDB 50053711 |
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
16. D(1) dopamine receptor (Protein Group)
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which activate adenylyl cyclase.
- Included Proteins:
- P21728 , P21918
References
- Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA: Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005 Mar 24;48(6):1709-12. [15771415 ]
- Shahid M, Walker GB, Zorn SH, Wong EH: Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009 Jan;23(1):65-73. doi: 10.1177/0269881107082944. Epub 2008 Feb 28. [18308814 ]
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which activate adenylyl cyclase.
- Gene Name:
- DRD5
- Uniprot ID:
- P21918
- Molecular Weight:
- 52950.5 Da
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO: Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res. 1999 May 4;37(1):107-22. [10227113 ]
- General Function:
- G-protein coupled acetylcholine receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is adenylate cyclase inhibition. Signaling promotes phospholipase C activity, leading to the release of inositol trisphosphate (IP3); this then triggers calcium ion release into the cytosol.
- Gene Name:
- CHRM2
- Uniprot ID:
- P08172
- Molecular Weight:
- 51714.605 Da
References
- General Function:
- Receptor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM3
- Uniprot ID:
- P20309
- Molecular Weight:
- 66127.445 Da
References
- General Function:
- Guanyl-nucleotide exchange factor activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is inhibition of adenylate cyclase.
- Gene Name:
- CHRM4
- Uniprot ID:
- P08173
- Molecular Weight:
- 53048.65 Da
References
- General Function:
- Phosphatidylinositol phospholipase c activity
- Specific Function:
- The muscarinic acetylcholine receptor mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Primary transducing effect is Pi turnover.
- Gene Name:
- CHRM5
- Uniprot ID:
- P08912
- Molecular Weight:
- 60073.205 Da
References
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for ergot alkaloid derivatives, various anxiolytic and antidepressant drugs and other psychoactive substances, such as lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity. Arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Regulates the release of 5-hydroxytryptamine, dopamine and acetylcholine in the brain, and thereby affects neural activity, nociceptive processing, pain perception, mood and behavior. Besides, plays a role in vasoconstriction of cerebral arteries.
- Gene Name:
- HTR1B
- Uniprot ID:
- P28222
- Molecular Weight:
- 43567.535 Da
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for ergot alkaloid derivatives, various anxiolytic and antidepressant drugs and other psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity. Regulates the release of 5-hydroxytryptamine in the brain, and thereby affects neural activity. May also play a role in regulating the release of other neurotransmitters. May play a role in vasoconstriction.
- Gene Name:
- HTR1D
- Uniprot ID:
- P28221
- Molecular Weight:
- 41906.38 Da
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various alkaloids and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity.
- Gene Name:
- HTR1E
- Uniprot ID:
- P28566
- Molecular Weight:
- 41681.57 Da
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various ergot alkaloid derivatives and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling activates a phosphatidylinositol-calcium second messenger system that modulates the activity of phosphatidylinositol 3-kinase and down-stream signaling cascades and promotes the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of dopamine and 5-hydroxytryptamine release, 5-hydroxytryptamine uptake and in the regulation of extracellular dopamine and 5-hydroxytryptamine levels, and thereby affects neural activity. May play a role in the perception of pain. Plays a role in the regulation of behavior, including impulsive behavior. Required for normal proliferation of embryonic cardiac myocytes and normal heart development. Protects cardiomyocytes against apoptosis. Plays a role in the adaptation of pulmonary arteries to chronic hypoxia. Plays a role in vasoconstriction. Required for normal osteoblast function and proliferation, and for maintaining normal bone density. Required for normal proliferation of the interstitial cells of Cajal in the intestine.
- Gene Name:
- HTR2B
- Uniprot ID:
- P41595
- Molecular Weight:
- 54297.41 Da
References
- Shahid M, Walker GB, Zorn SH, Wong EH: Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009 Jan;23(1):65-73. doi: 10.1177/0269881107082944. Epub 2008 Feb 28. [18308814 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. This receptor is a ligand-gated ion channel, which when activated causes fast, depolarizing responses in neurons. It is a cation-specific, but otherwise relatively nonselective, ion channel.
- Gene Name:
- HTR3A
- Uniprot ID:
- P46098
- Molecular Weight:
- 55279.835 Da
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins.
- Gene Name:
- HTR5A
- Uniprot ID:
- P47898
- Molecular Weight:
- 40254.69 Da
References
- Shahid M, Walker GB, Zorn SH, Wong EH: Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009 Jan;23(1):65-73. doi: 10.1177/0269881107082944. Epub 2008 Feb 28. [18308814 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- This alpha-adrenergic receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Its effect is mediated by G(q) and G(11) proteins. Nuclear ADRA1A-ADRA1B heterooligomers regulate phenylephrine (PE)-stimulated ERK signaling in cardiac myocytes.
- Gene Name:
- ADRA1B
- Uniprot ID:
- P35368
- Molecular Weight:
- 56835.375 Da
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- General Function:
- Epinephrine binding
- Specific Function:
- Alpha-2 adrenergic receptors mediate the catecholamine-induced inhibition of adenylate cyclase through the action of G proteins. The rank order of potency for agonists of this receptor is clonidine > norepinephrine > epinephrine = oxymetazoline > dopamine > p-tyramine = phenylephrine > serotonin > p-synephrine / p-octopamine. For antagonists, the rank order is yohimbine > chlorpromazine > phentolamine > mianserine > spiperone > prazosin > alprenolol > propanolol > pindolol.
- Gene Name:
- ADRA2B
- Uniprot ID:
- P18089
- Molecular Weight:
- 49565.8 Da
References
- Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008 Jan;13(1):27-35. Epub 2007 Sep 11. [17848919 ]
30. Beta adrenergic receptor (Protein Group)
- General Function:
- Receptor signaling protein activity
- Specific Function:
- Beta-adrenergic receptors mediate the catecholamine-induced activation of adenylate cyclase through the action of G proteins. This receptor binds epinephrine and norepinephrine with approximately equal affinity. Mediates Ras activation through G(s)-alpha- and cAMP-mediated signaling.
- Included Proteins:
- P08588 , P07550 , P13945
References
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT: Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996 Feb;14(2):87-96. [8822531 ]
31. D(2L) dopamine receptor
References
- Shahid M, Walker GB, Zorn SH, Wong EH: Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009 Jan;23(1):65-73. doi: 10.1177/0269881107082944. Epub 2008 Feb 28. [18308814 ]
32. D(2S) dopamine receptor
References
- Shahid M, Walker GB, Zorn SH, Wong EH: Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009 Jan;23(1):65-73. doi: 10.1177/0269881107082944. Epub 2008 Feb 28. [18308814 ]
33. GABA-A receptor (anion channel) (Protein Group)
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Included Proteins:
- P14867 , P47869 , P34903 , P48169 , P31644 , Q16445 , P18505 , P47870 , P28472 , O14764 , P78334 , Q8N1C3 , P18507 , Q99928 , O00591 , Q9UN88
References
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT: Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996 Feb;14(2):87-96. [8822531 ]
- General Function:
- Histamine receptor activity
- Specific Function:
- The H2 subclass of histamine receptors mediates gastric acid secretion. Also appears to regulate gastrointestinal motility and intestinal secretion. Possible role in regulating cell growth and differentiation. The activity of this receptor is mediated by G proteins which activate adenylyl cyclase and, through a separate G protein-dependent mechanism, the phosphoinositide/protein kinase (PKC) signaling pathway (By similarity).
- Gene Name:
- HRH2
- Uniprot ID:
- P25021
- Molecular Weight:
- 40097.65 Da
References
- Shahid M, Walker GB, Zorn SH, Wong EH: Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009 Jan;23(1):65-73. doi: 10.1177/0269881107082944. Epub 2008 Feb 28. [18308814 ]
- General Function:
- Histamine receptor activity
- Specific Function:
- The H4 subclass of histamine receptors could mediate the histamine signals in peripheral tissues. Displays a significant level of constitutive activity (spontaneous activity in the absence of agonist).
- Gene Name:
- HRH4
- Uniprot ID:
- Q9H3N8
- Molecular Weight:
- 44495.375 Da
References
- Shahid M, Walker GB, Zorn SH, Wong EH: Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009 Jan;23(1):65-73. doi: 10.1177/0269881107082944. Epub 2008 Feb 28. [18308814 ]
- General Function:
- Serotonin:sodium symporter activity
- Specific Function:
- Serotonin transporter whose primary function in the central nervous system involves the regulation of serotonergic signaling via transport of serotonin molecules from the synaptic cleft back into the pre-synaptic terminal for re-utilization. Plays a key role in mediating regulation of the availability of serotonin to other receptors of serotonergic systems. Terminates the action of serotonin and recycles it in a sodium-dependent manner.
- Gene Name:
- SLC6A4
- Uniprot ID:
- P31645
- Molecular Weight:
- 70324.165 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >15 uM | Not Available | BindingDB 50053711 |
References
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EV, Jackson T, Khan A, Roth BL: Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008 Aug 1;16(15):7291-301. doi: 10.1016/j.bmc.2008.06.030. Epub 2008 Jun 20. [18595716 ]