Indomethacin (T3D2753)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:26:37 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:51 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2753 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Indomethacin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Indomethacin is a non-steroidal antiinflammatory agent (NSAIA) with antiinflammatory, analgesic and antipyretic activity. Its pharmacological effect is thought to be mediated through inhibition of the enzyme cyclooxygenase (COX), the enzyme responsible for catalyzes the rate-limiting step in prostaglandin synthesis via the arachidonic acid pathway. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
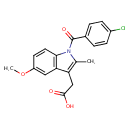
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C19H16ClNO4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 357.788 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 357.077 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 53-86-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | indomethacin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | COC1=CC2=C(C=C1)N(C(=O)C1=CC=C(Cl)C=C1)C(C)=C2CC(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=CGIGDMFJXJATDK-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as benzoylindoles. These are organic compounds containing an indole attached to a benzoyl moiety through the acyl group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Indoles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Benzoylindoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Benzoylindoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Parenteral(intravenous); enteral(rectal); oral. Bioavailability is approximately 100% following oral administration and 80-90% following rectal administration. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Antiinflammatory effects of Indomethacin are believed to be due to inhibition of cylooxygenase in platelets which leads to the blockage of prostaglandin synthesis. Antipyretic effects may be due to action on the hypothalamus, resulting in an increased peripheral blood flow, vasodilation, and subsequent heat dissipation. Indomethacin is a prostaglandin G/H synthase (also known as cyclooxygenase or COX) inhibitor that acts on both prostaglandin G/H synthase 1 and 2 (COX-1 and -2). Prostaglandin G/H synthase catalyzes the conversion of arachidonic acid to a number of prostaglandins involved in fever, pain, swelling, inflammation, and platelet aggregation. Indomethacin antagonizes COX by binding to the upper portion of the active site, preventing its substrate, arachidonic acid, from entering the active site. Indomethacin, unlike other NSAIDs, also inhibits phospholipase A2, the enzyme responsible for releasing arachidonic acid from phospholipids. Indomethacin is more selective for COX-1 than COX-2, which accounts for its increased adverse gastric effects relative to other NSAIDs. COX-1 is required for maintaining the protective gastric mucosal layer. The analgesic, antipyretic and anti-inflammatory effects of indomethacin occur as a result of decreased prostaglandin synthesis. Its antipyretic effects may be due to action on the hypothalamus, resulting in an increased peripheral blood flow, vasodilation, and subsequent heat dissipation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Hepatic. Route of Elimination: Indomethacin is eliminated via renal excretion, metabolism, and biliary excretion. Half Life: 4.5 hours | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 50 mg/kg (oral, mice) (based on 14 day mortality response) LD50: 12 mg/kg (oral, rat) (based on 14 day mortality response) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For moderate to severe rheumatoid arthritis including acute flares of chronic disease, ankylosing spondylitis, osteoarthritis, acute painful shoulder (bursitis and/or tendinitis) and acute gouty arthritis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | The following symptoms may be observed following overdosage: nausea, vomiting, intense headache, dizziness, mental confusion, disorientation, or lethargy. There have been reports of paresthesias, numbness, and convulsions. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Treatment is symptomatic and supportive. The stomach should be emptied as quickly as possible if the ingestion is recent. If vomiting has not occurred spontaneously, the patient should be induced to vomit with syrup of ipecac. If the patient is unable to vomit, gastric lavage should be performed. Once the stomach has been emptied, 25 or 50 g of activated charcoal may be given. Depending on the condition of the patient, close medical observation and nursing care may be required. The patient should be followed for several days because gastrointestinal ulceration and hemorrhage have been reported as adverse reactions of indomethacin. Use of antacids may be helpful. (8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00328 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB14473 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 3715 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 3584 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C01926 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 5918 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | CPD-10545 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Indometacin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | IMN | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Indomethacin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Hubertus L. Regtop, John R. Biffin, “Preparation of divalent metal salts of indomethacin.” U.S. Patent US5310936, issued November, 1917. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear receptor that binds peroxisome proliferators such as hypolipidemic drugs and fatty acids. Once activated by a ligand, the nuclear receptor binds to DNA specific PPAR response elements (PPRE) and modulates the transcription of its target genes, such as acyl-CoA oxidase. It therefore controls the peroxisomal beta-oxidation pathway of fatty acids. Key regulator of adipocyte differentiation and glucose homeostasis. ARF6 acts as a key regulator of the tissue-specific adipocyte P2 (aP2) enhancer. Acts as a critical regulator of gut homeostasis by suppressing NF-kappa-B-mediated proinflammatory responses. Plays a role in the regulation of cardiovascular circadian rhythms by regulating the transcription of ARNTL/BMAL1 in the blood vessels (By similarity).
- Gene Name:
- PPARG
- Uniprot ID:
- P37231
- Molecular Weight:
- 57619.58 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| AC50 | 2.53 uM | Tox21_PPARg_BLA_Agonist_ratio | Tox21/NCGC |
References
- Cho MC, Lee HS, Kim JH, Choe YK, Hong JT, Paik SG, Yoon DY: A simple ELISA for screening ligands of peroxisome proliferator-activated receptor-gamma. J Biochem Mol Biol. 2003 Mar 31;36(2):207-13. [12689521 ]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA: Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997 Feb 7;272(6):3406-10. [9013583 ]
- Sipes NS, Martin MT, Kothiya P, Reif DM, Judson RS, Richard AM, Houck KA, Dix DJ, Kavlock RJ, Knudsen TB: Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem Res Toxicol. 2013 Jun 17;26(6):878-95. doi: 10.1021/tx400021f. Epub 2013 May 16. [23611293 ]
- General Function:
- Prostaglandin-endoperoxide synthase activity
- Specific Function:
- Converts arachidonate to prostaglandin H2 (PGH2), a committed step in prostanoid synthesis. Constitutively expressed in some tissues in physiological conditions, such as the endothelium, kidney and brain, and in pathological conditions, such as in cancer. PTGS2 is responsible for production of inflammatory prostaglandins. Up-regulation of PTGS2 is also associated with increased cell adhesion, phenotypic changes, resistance to apoptosis and tumor angiogenesis. In cancer cells, PTGS2 is a key step in the production of prostaglandin E2 (PGE2), which plays important roles in modulating motility, proliferation and resistance to apoptosis.
- Gene Name:
- PTGS2
- Uniprot ID:
- P35354
- Molecular Weight:
- 68995.625 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.37 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.4 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.44 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.6 uM | Not Available | BindingDB 17638 |
| IC50 | 0.0059 uM | Not Available | BindingDB 17638 |
| IC50 | 0.009 uM | Not Available | BindingDB 17638 |
| IC50 | 0.01 uM | Not Available | BindingDB 17638 |
| IC50 | 0.026 uM | Not Available | BindingDB 17638 |
| IC50 | 0.03 uM | Not Available | BindingDB 17638 |
| IC50 | 0.036 uM | Not Available | BindingDB 17638 |
| IC50 | 0.04 uM | Not Available | BindingDB 17638 |
| IC50 | 0.048 uM | Not Available | BindingDB 17638 |
| IC50 | 0.049 uM | Not Available | BindingDB 17638 |
| IC50 | 0.22 uM | Not Available | BindingDB 17638 |
| IC50 | 0.4 uM | Not Available | BindingDB 17638 |
| IC50 | 0.41 uM | Not Available | BindingDB 17638 |
| IC50 | 0.44 uM | Not Available | BindingDB 17638 |
| IC50 | 0.46 uM | Not Available | BindingDB 17638 |
| IC50 | 0.5 uM | Not Available | BindingDB 17638 |
| IC50 | 0.56 uM | Not Available | BindingDB 17638 |
| IC50 | 0.6 uM | Not Available | BindingDB 17638 |
| IC50 | 0.63 uM | Not Available | BindingDB 17638 |
| IC50 | 0.64 uM | Not Available | BindingDB 17638 |
| IC50 | 0.67 uM | Not Available | BindingDB 17638 |
| IC50 | 0.7 uM | Not Available | BindingDB 17638 |
| IC50 | 0.73 uM | Not Available | BindingDB 17638 |
| IC50 | 0.75 uM | Not Available | BindingDB 17638 |
| IC50 | 0.9 uM | Not Available | BindingDB 17638 |
| IC50 | 1.2 uM | Not Available | BindingDB 17638 |
| IC50 | 1.9 uM | Not Available | BindingDB 17638 |
| IC50 | 2.38 uM | Not Available | BindingDB 17638 |
| IC50 | 2.4 uM | Not Available | BindingDB 17638 |
| IC50 | 2.63 uM | Not Available | BindingDB 17638 |
| IC50 | 3.9 uM | Not Available | BindingDB 17638 |
| IC50 | 5.7 uM | Not Available | BindingDB 17638 |
| IC50 | 5.8 uM | Not Available | BindingDB 17638 |
| IC50 | 6.9 uM | Not Available | BindingDB 17638 |
| IC50 | 7.8 uM | Not Available | BindingDB 17638 |
| IC50 | 7.81 uM | Not Available | BindingDB 17638 |
| IC50 | 11 uM | Not Available | BindingDB 17638 |
| IC50 | 17 uM | Not Available | BindingDB 17638 |
| IC50 | 18 uM | Not Available | BindingDB 17638 |
| IC50 | 18.3 uM | Not Available | BindingDB 17638 |
| IC50 | 20 uM | Not Available | BindingDB 17638 |
| IC50 | 35.2 uM | Not Available | BindingDB 17638 |
| IC50 | 180 uM | Not Available | BindingDB 17638 |
References
- Jerde TJ, Calamon-Dixon JL, Bjorling DE, Nakada SY: Celecoxib inhibits ureteral contractility and prostanoid release. Urology. 2005 Jan;65(1):185-90. [15667901 ]
- Pilane CM, Labelle EF: Nitric oxide stimulated vascular smooth muscle cells undergo apoptosis induced in part by arachidonic acid derived eicosanoids. J Cell Physiol. 2005 Aug;204(2):423-7. [15668944 ]
- Zhang GS, Fu YB, Xia M: [Proliferation inhibition effect of indomethacin on CML cells associated with down-regulation of phosphorylated STAT1/STAT5 and inhibition of COX-2 expression]. Zhonghua Xue Ye Xue Za Zhi. 2004 Dec;25(12):732-5. [15730717 ]
- Armstrong PJ, Franklin DP, Carey DJ, Elmore JR: Suppression of experimental aortic aneurysms: comparison of inducible nitric oxide synthase and cyclooxygenase inhibitors. Ann Vasc Surg. 2005 Mar;19(2):248-57. [15770365 ]
- Yokota A, Taniguchi M, Takahira Y, Tanaka A, Takeuchi K: Rofecoxib produces intestinal but not gastric damage in the presence of a low dose of indomethacin in rats. J Pharmacol Exp Ther. 2005 Jul;314(1):302-9. Epub 2005 Apr 14. [15831440 ]
- Palomer A, Cabre F, Pascual J, Campos J, Trujillo MA, Entrena A, Gallo MA, Garcia L, Mauleon D, Espinosa A: Identification of novel cyclooxygenase-2 selective inhibitors using pharmacophore models. J Med Chem. 2002 Mar 28;45(7):1402-11. [11906281 ]
- Almansa C, de Arriba AF, Cavalcanti FL, Gomez LA, Miralles A, Merlos M, Garcia-Rafanell J, Forn J: Synthesis and SAR of a new series of COX-2-selective inhibitors: pyrazolo[1,5-a]pyrimidines. J Med Chem. 2001 Feb 1;44(3):350-61. [11462976 ]
- Almansa C, Alfon J, de Arriba AF, Cavalcanti FL, Escamilla I, Gomez LA, Miralles A, Soliva R, Bartroli J, Carceller E, Merlos M, Garcia-Rafanell J: Synthesis and structure-activity relationship of a new series of COX-2 selective inhibitors: 1,5-diarylimidazoles. J Med Chem. 2003 Jul 31;46(16):3463-75. [12877584 ]
- Blobaum AL, Marnett LJ: Structural and functional basis of cyclooxygenase inhibition. J Med Chem. 2007 Apr 5;50(7):1425-41. Epub 2007 Mar 7. [17341061 ]
- Hieke M, Ness J, Steri R, Dittrich M, Greiner C, Werz O, Baumann K, Schubert-Zsilavecz M, Weggen S, Zettl H: Design, synthesis, and biological evaluation of a novel class of gamma-secretase modulators with PPARgamma activity. J Med Chem. 2010 Jun 24;53(12):4691-700. doi: 10.1021/jm1003073. [20503989 ]
- Hieke M, Ness J, Steri R, Greiner C, Werz O, Schubert-Zsilavecz M, Weggen S, Zettl H: SAR studies of acidic dual gamma-secretase/PPARgamma modulators. Bioorg Med Chem. 2011 Sep 15;19(18):5372-82. doi: 10.1016/j.bmc.2011.08.003. Epub 2011 Aug 6. [21873070 ]
- Friesen RW, Brideau C, Chan CC, Charleson S, Deschenes D, Dube D, Ethier D, Fortin R, Gauthier JY, Girard Y, Gordon R, Greig GM, Riendeau D, Savoie C, Wang Z, Wong E, Visco D, Xu LJ, Young RN: 2-Pyridinyl-3-(4-methylsulfonyl)phenylpyridines: selective and orally active cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett. 1998 Oct 6;8(19):2777-82. [9873621 ]
- Li CS, Black WC, Brideau C, Chan CC, Charleson S, Cromlish WA, Claveau D, Gauthier JY, Gordon R, Greig G, Grimm E, Guay J, Lau CK, Riendeau D, Therien M, Visco DM, Wong E, Xu L, Prasit P: A new structural variation on the methanesulfonylphenyl class of selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett. 1999 Nov 15;9(22):3181-6. [10576684 ]
- Lau CK, Brideau C, Chan CC, Charleson S, Cromlish WA, Ethier D, Gauthier JY, Gordon R, Guay J, Kargman S, Li CS, Prasit P, Riendeau D, Therien M, Visco DM, Xu L: Synthesis and biological evaluation of 3-heteroaryloxy-4-phenyl-2(5H)-furanones as selective COX-2 inhibitors. Bioorg Med Chem Lett. 1999 Nov 15;9(22):3187-92. [10576685 ]
- Liu T, Lin Y, Wen X, Jorissen RN, Gilson MK: BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007 Jan;35(Database issue):D198-201. Epub 2006 Dec 1. [17145705 ]
- Prasit P, Wang Z, Brideau C, Chan CC, Charleson S, Cromlish W, Ethier D, Evans JF, Ford-Hutchinson AW, Gauthier JY, Gordon R, Guay J, Gresser M, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini J, O'Neill GP, Ouellet M, Percival MD, Perrier H, Riendeau D, Rodger I, Zamboni R, et al.: The discovery of rofecoxib, [MK 966, Vioxx, 4-(4'-methylsulfonylphenyl)-3-phenyl-2(5H)-furanone], an orally active cyclooxygenase-2-inhibitor. Bioorg Med Chem Lett. 1999 Jul 5;9(13):1773-8. [10406640 ]
- Woods KW, McCroskey RW, Michaelides MR, Wada CK, Hulkower KI, Bell RL: Thiazole analogues of the NSAID indomethacin as selective COX-2 inhibitors. Bioorg Med Chem Lett. 2001 May 21;11(10):1325-8. [11392547 ]
- Tsai WJ, Shiao YJ, Lin SJ, Chiou WF, Lin LC, Yang TH, Teng CM, Wu TS, Yang LM: Selective COX-2 inhibitors. Part 1: synthesis and biological evaluation of phenylazobenzenesulfonamides. Bioorg Med Chem Lett. 2006 Sep 1;16(17):4440-3. Epub 2006 Jun 30. [16814546 ]
- Walters MJ, Blobaum AL, Kingsley PJ, Felts AS, Sulikowski GA, Marnett LJ: The influence of double bond geometry in the inhibition of cyclooxygenases by sulindac derivatives. Bioorg Med Chem Lett. 2009 Jun 15;19(12):3271-4. doi: 10.1016/j.bmcl.2009.04.078. Epub 2009 Apr 23. [19427206 ]
- Rambabu D, Mulakayala N, Ismail, Kumar KR, Kumar GP, Mulakayala C, Kumar CS, Kalle AM, Rao MV, Oruganti S, Pal M: Synthesis and pharmacological evaluation of N-substituted 2-(2-oxo-2H-chromen-4-yloxy)propanamide as cyclooxygenase inhibitors. Bioorg Med Chem Lett. 2012 Nov 1;22(21):6745-9. doi: 10.1016/j.bmcl.2012.08.082. Epub 2012 Aug 31. [23010270 ]
- Puig C, Crespo MI, Godessart N, Feixas J, Ibarzo J, Jimenez JM, Soca L, Cardelus I, Heredia A, Miralpeix M, Puig J, Beleta J, Huerta JM, Lopez M, Segarra V, Ryder H, Palacios JM: Synthesis and biological evaluation of 3,4-diaryloxazolones: A new class of orally active cyclooxygenase-2 inhibitors. J Med Chem. 2000 Jan 27;43(2):214-23. [10649977 ]
- Leblanc Y, Roy P, Boyce S, Brideau C, Chan CC, Charleson S, Gordon R, Grimm E, Guay J, Leger S, Li CS, Riendeau D, Visco D, Wang Z, Webb J, Xu LJ, Prasit P: SAR in the alkoxy lactone series: the discovery of DFP, a potent and orally active COX-2 inhibitor. Bioorg Med Chem Lett. 1999 Aug 2;9(15):2207-12. [10465547 ]
- Bosch J, Roca T, Catena JL, Llorens O, Perez JJ, Lagunas C, Fernandez AG, Miquel I, Fernandez-Serrat A, Farrerons C: Synthesis and biological evaluation of 1,3,4-triaryl-3-pyrrolin-2-ones, a new class of selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett. 2000 Aug 7;10(15):1745-8. [10937738 ]
- Riendeau D, Salem M, Styhler A, Ouellet M, Mancini JA, Li CS: Evaluation of loxoprofen and its alcohol metabolites for potency and selectivity of inhibition of cyclooxygenase-2. Bioorg Med Chem Lett. 2004 Mar 8;14(5):1201-3. [14980665 ]
- Dube D, Brideau C, Deschenes D, Fortin R, Friesen RW, Gordon R, Girard Y, Riendeau D, Savoie C, Chan CC: 2-heterosubstituted-3-(4-methylsulfonyl)phenyl-5-trifluoromethyl pyridines as selective and orally active cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett. 1999 Jun 21;9(12):1715-20. [10397507 ]
- Bridoux A, Millet R, Pommery J, Pommery N, Henichart JP: Synthesis and biological activity of N-aroyl-tetrahydro-gamma-carbolines. Bioorg Med Chem. 2010 Jun 1;18(11):3910-24. doi: 10.1016/j.bmc.2010.04.034. Epub 2010 Apr 18. [20451397 ]
- Hansen FK, Khankischpur M, Tolaymat I, Mesaros R, Dannhardt G, Geffken D: Efficient synthesis and 5-LOX/COX-inhibitory activity of some 3-hydroxybenzo[b]thiophene-2-carboxylic acid derivatives. Bioorg Med Chem Lett. 2012 Aug 1;22(15):5031-4. doi: 10.1016/j.bmcl.2012.06.012. Epub 2012 Jun 13. [22749420 ]
- Kolasa T, Brooks CD, Rodriques KE, Summers JB, Dellaria JF, Hulkower KI, Bouska J, Bell RL, Carter GW: Nonsteroidal anti-inflammatory drugs as scaffolds for the design of 5-lipoxygenase inhibitors. J Med Chem. 1997 Feb 28;40(5):819-24. [9057869 ]
- Vardanyan R, Vijay G, Nichol GS, Liu L, Kumarasinghe I, Davis P, Vanderah T, Porreca F, Lai J, Hruby VJ: Synthesis and investigations of double-pharmacophore ligands for treatment of chronic and neuropathic pain. Bioorg Med Chem. 2009 Jul 15;17(14):5044-53. doi: 10.1016/j.bmc.2009.05.065. Epub 2009 Jun 2. [19540763 ]
- Khan KM, Ambreen N, Mughal UR, Jalil S, Perveen S, Choudhary MI: 3-Formylchromones: potential antiinflammatory agents. Eur J Med Chem. 2010 Sep;45(9):4058-64. doi: 10.1016/j.ejmech.2010.05.065. Epub 2010 Jun 8. [20576329 ]
- Wey SJ, Augustyniak ME, Cochran ED, Ellis JL, Fang X, Garvey DS, Janero DR, Letts LG, Martino AM, Melim TL, Murty MG, Richardson SK, Schroeder JD, Selig WM, Trocha AM, Wexler RS, Young DV, Zemtseva IS, Zifcak BM: Structure-based design, synthesis, and biological evaluation of indomethacin derivatives as cyclooxygenase-2 inhibiting nitric oxide donors. J Med Chem. 2007 Dec 13;50(25):6367-82. Epub 2007 Nov 10. [17994684 ]
- Kalgutkar AS, Crews BC, Rowlinson SW, Marnett AB, Kozak KR, Remmel RP, Marnett LJ: Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):925-30. [10639181 ]
- Kalgutkar AS, Marnett AB, Crews BC, Remmel RP, Marnett LJ: Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J Med Chem. 2000 Jul 27;43(15):2860-70. [10956194 ]
- Scholz M, Blobaum AL, Marnett LJ, Hey-Hawkins E: Synthesis and evaluation of carbaborane derivatives of indomethacin as cyclooxygenase inhibitors. Bioorg Med Chem. 2011 May 15;19(10):3242-8. doi: 10.1016/j.bmc.2011.03.054. Epub 2011 Mar 27. [21524587 ]
- Liedtke AJ, Crews BC, Daniel CM, Blobaum AL, Kingsley PJ, Ghebreselasie K, Marnett LJ: Cyclooxygenase-1-selective inhibitors based on the (E)-2'-des-methyl-sulindac sulfide scaffold. J Med Chem. 2012 Mar 8;55(5):2287-300. doi: 10.1021/jm201528b. Epub 2012 Feb 14. [22263894 ]
- Li JJ, Anderson GD, Burton EG, Cogburn JN, Collins JT, Garland DJ, Gregory SA, Huang HC, Isakson PC, Koboldt CM, et al.: 1,2-Diarylcyclopentenes as selective cyclooxygenase-2 inhibitors and orally active anti-inflammatory agents. J Med Chem. 1995 Oct 27;38(22):4570-8. [7473585 ]
- Li JJ, Norton MB, Reinhard EJ, Anderson GD, Gregory SA, Isakson PC, Koboldt CM, Masferrer JL, Perkins WE, Seibert K, Zhang Y, Zweifel BS, Reitz DB: Novel terphenyls as selective cyclooxygenase-2 inhibitors and orally active anti-inflammatory agents. J Med Chem. 1996 Apr 26;39(9):1846-56. [8627608 ]
- Khanna IK, Weier RM, Yu Y, Xu XD, Koszyk FJ, Collins PW, Koboldt CM, Veenhuizen AW, Perkins WE, Casler JJ, Masferrer JL, Zhang YY, Gregory SA, Seibert K, Isakson PC: 1,2-Diarylimidazoles as potent, cyclooxygenase-2 selective, and orally active antiinflammatory agents. J Med Chem. 1997 May 23;40(11):1634-47. [9171873 ]
- Khanna IK, Yu Y, Huff RM, Weier RM, Xu X, Koszyk FJ, Collins PW, Cogburn JN, Isakson PC, Koboldt CM, Masferrer JL, Perkins WE, Seibert K, Veenhuizen AW, Yuan J, Yang DC, Zhang YY: Selective cyclooxygenase-2 inhibitors: heteroaryl modified 1,2-diarylimidazoles are potent, orally active antiinflammatory agents. J Med Chem. 2000 Aug 10;43(16):3168-85. [10956225 ]
- Reitz DB, Li JJ, Norton MB, Reinhard EJ, Collins JT, Anderson GD, Gregory SA, Koboldt CM, Perkins WE, Seibert K, et al.: Selective cyclooxygenase inhibitors: novel 1,2-diarylcyclopentenes are potent and orally active COX-2 inhibitors. J Med Chem. 1994 Nov 11;37(23):3878-81. [7966148 ]
- Hosek J, Bartos M, Chudik S, Dall'Acqua S, Innocenti G, Kartal M, Kokoska L, Kollar P, Kutil Z, Landa P, Marek R, Zavalova V, Zemlicka M, Smejkal K: Natural compound cudraflavone B shows promising anti-inflammatory properties in vitro. J Nat Prod. 2011 Apr 25;74(4):614-9. doi: 10.1021/np100638h. Epub 2011 Feb 14. [21319773 ]
- Hashimoto H, Imamura K, Haruta J, Wakitani K: 4-(4-cycloalkyl/aryl-oxazol-5-yl)benzenesulfonamides as selective cyclooxygenase-2 inhibitors: enhancement of the selectivity by introduction of a fluorine atom and identification of a potent, highly selective, and orally active COX-2 inhibitor JTE-522(1). J Med Chem. 2002 Mar 28;45(7):1511-7. [11906292 ]
- Hashimoto H, Maeda K, Ozawa K, Haruta J, Wakitani K: 4-Aryl/cycloalkyl-5-phenyloxazole derivatives as selective COX-2 inhibitors. Bioorg Med Chem Lett. 2002 Jan 7;12(1):65-8. [11738574 ]
- Bekhit AA, Ashour HM, Abdel Ghany YS, Bekhit Ael-D, Baraka A: Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur J Med Chem. 2008 Mar;43(3):456-63. Epub 2007 Apr 14. [17532544 ]
- Bekhit AA, Ashour HM, Bekhit Ael-D, Abdel-Rahman HM, Bekhit SA: Synthesis of some pyrazolyl benzenesulfonamide derivatives as dual anti-inflammatory antimicrobial agents. J Enzyme Inhib Med Chem. 2009 Feb;24(1):296-309. doi: 10.1080/14756360802188404 . [18951238 ]
- Bekhit AA, Fahmy HT, Rostom SA, El-Din A Bekhit A: Synthesis and biological evaluation of some thiazolylpyrazole derivatives as dual anti-inflammatory antimicrobial agents. Eur J Med Chem. 2010 Dec;45(12):6027-38. doi: 10.1016/j.ejmech.2010.10.001. Epub 2010 Oct 20. [20970223 ]
- Larsson J, Gottfries J, Bohlin L, Backlund A: Expanding the ChemGPS chemical space with natural products. J Nat Prod. 2005 Jul;68(7):985-91. [16038536 ]
- Jain S, Tran S, El Gendy MA, Kashfi K, Jurasz P, Velazquez-Martinez CA: Nitric oxide release is not required to decrease the ulcerogenic profile of nonsteroidal anti-inflammatory drugs. J Med Chem. 2012 Jan 26;55(2):688-96. doi: 10.1021/jm200973j. Epub 2012 Jan 10. [22148253 ]
- Song Y, Connor DT, Doubleday R, Sorenson RJ, Sercel AD, Unangst PC, Roth BD, Gilbertsen RB, Chan K, Schrier DJ, Guglietta A, Bornemeier DA, Dyer RD: Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 1. Thiazolone and oxazolone series. J Med Chem. 1999 Apr 8;42(7):1151-60. [10197959 ]
- Song Y, Connor DT, Sercel AD, Sorenson RJ, Doubleday R, Unangst PC, Roth BD, Beylin VG, Gilbertsen RB, Chan K, Schrier DJ, Guglietta A, Bornemeier DA, Dyer RD: Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 2. 1,3,4- and 1,2,4-thiadiazole series. J Med Chem. 1999 Apr 8;42(7):1161-9. [10197960 ]
- Rios MY, Aguilar-Guadarrama AB: Nitrogen-containing phorbol esters from Croton ciliatoglandulifer and their effects on cyclooxygenases-1 and -2. J Nat Prod. 2006 Jun;69(6):887-90. [16792405 ]
- Huang Z, Velazquez CA, Abdellatif KR, Chowdhury MA, Reisz JA, DuMond JF, King SB, Knaus EE: Ethanesulfohydroxamic acid ester prodrugs of nonsteroidal anti-inflammatory drugs (NSAIDs): synthesis, nitric oxide and nitroxyl release, cyclooxygenase inhibition, anti-inflammatory, and ulcerogenicity index studies. J Med Chem. 2011 Mar 10;54(5):1356-64. doi: 10.1021/jm101403g. Epub 2011 Jan 31. [21280601 ]
- Kaur J, Bhardwaj A, Huang Z, Knaus EE: N-1 and C-3 substituted indole Schiff bases as selective COX-2 inhibitors: synthesis and biological evaluation. Bioorg Med Chem Lett. 2012 Mar 15;22(6):2154-9. doi: 10.1016/j.bmcl.2012.01.130. Epub 2012 Feb 6. [22361134 ]
- Pal M, Madan M, Padakanti S, Pattabiraman VR, Kalleda S, Vanguri A, Mullangi R, Mamidi NV, Casturi SR, Malde A, Gopalakrishnan B, Yeleswarapu KR: Synthesis and cyclooxygenase-2 inhibiting property of 1,5-diarylpyrazoles with substituted benzenesulfonamide moiety as pharmacophore: Preparation of sodium salt for injectable formulation. J Med Chem. 2003 Sep 11;46(19):3975-84. [12954051 ]
- Singh SK, Vobbalareddy S, Shivaramakrishna S, Krishnamraju A, Rajjak SA, Casturi SR, Akhila V, Rao YK: Methanesulfonamide group at position-4 of the C-5-phenyl ring of 1,5-diarylpyrazole affords a potent class of cyclooxygenase-2 (COX-2) inhibitors. Bioorg Med Chem Lett. 2004 Apr 5;14(7):1683-8. [15026050 ]
- Kakuta H, Zheng X, Oda H, Harada S, Sugimoto Y, Sasaki K, Tai A: Cyclooxygenase-1-selective inhibitors are attractive candidates for analgesics that do not cause gastric damage. design and in vitro/in vivo evaluation of a benzamide-type cyclooxygenase-1 selective inhibitor. J Med Chem. 2008 Apr 24;51(8):2400-11. doi: 10.1021/jm701191z. Epub 2008 Mar 26. [18363350 ]
- Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD: Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem. 2011 Apr 28;54(8):3037-50. doi: 10.1021/jm2001376. Epub 2011 Apr 5. [21434686 ]
- Unsal-Tan O, Ozadali K, Piskin K, Balkan A: Molecular modeling, synthesis and screening of some new 4-thiazolidinone derivatives with promising selective COX-2 inhibitory activity. Eur J Med Chem. 2012 Nov;57:59-64. doi: 10.1016/j.ejmech.2012.08.046. Epub 2012 Sep 7. [23047224 ]
- Fioravanti R, Bolasco A, Manna F, Rossi F, Orallo F, Ortuso F, Alcaro S, Cirilli R: Synthesis and biological evaluation of N-substituted-3,5-diphenyl-2-pyrazoline derivatives as cyclooxygenase (COX-2) inhibitors. Eur J Med Chem. 2010 Dec;45(12):6135-8. doi: 10.1016/j.ejmech.2010.10.005. Epub 2010 Oct 25. [20974503 ]
- Wilkerson WW, Copeland RA, Covington M, Trzaskos JM: Antiinflammatory 4,5-diarylpyrroles. 2. Activity as a function of cyclooxygenase-2 inhibition. J Med Chem. 1995 Sep 29;38(20):3895-901. [7562922 ]
- Cryer B, Feldman M: Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998 May;104(5):413-21. [9626023 ]
- Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Ford-Hutchinson AW, Forrest MJ, Gauthier JY, Gordon R, Gresser M, Guay J, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini J, O'Neill GP, Ouellet M, Patrick D, Percival MD, Perrier H, Prasit P, Rodger I, et al.: Rofecoxib [Vioxx, MK-0966; 4-(4'-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999 Aug;290(2):551-60. [10411562 ]
- Riendeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret JP, Friesen RW, Gordon R, Greig G, Guay J, Mancini J, Ouellet M, Wong E, Xu L, Boyce S, Visco D, Girard Y, Prasit P, Zamboni R, Rodger IW, Gresser M, Ford-Hutchinson AW, Young RN, Chan CC: Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther. 2001 Feb;296(2):558-66. [11160644 ]
- Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Falgueyret JP, Ford-Hutchinson AW, Gordon R, Greig G, Gresser M, Guay J, Kargman S, Leger S, Mancini JA, O'Neill G, Ouellet M, Rodger IW, Therien M, Wang Z, Webb JK, Wong E, Chan CC, et al.: Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997 May;121(1):105-17. [9146894 ]
- General Function:
- Prostaglandin-endoperoxide synthase activity
- Specific Function:
- Converts arachidonate to prostaglandin H2 (PGH2), a committed step in prostanoid synthesis. Involved in the constitutive production of prostanoids in particular in the stomach and platelets. In gastric epithelial cells, it is a key step in the generation of prostaglandins, such as prostaglandin E2 (PGE2), which plays an important role in cytoprotection. In platelets, it is involved in the generation of thromboxane A2 (TXA2), which promotes platelet activation and aggregation, vasoconstriction and proliferation of vascular smooth muscle cells.
- Gene Name:
- PTGS1
- Uniprot ID:
- P23219
- Molecular Weight:
- 68685.82 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.01 uM | Not Available | BindingDB 17638 |
| IC50 | 0.02 uM | Not Available | BindingDB 17638 |
| IC50 | 0.044 uM | Not Available | BindingDB 17638 |
| IC50 | 0.05 uM | Not Available | BindingDB 17638 |
| IC50 | 0.1 uM | Not Available | BindingDB 17638 |
| IC50 | 0.15 uM | Not Available | BindingDB 17638 |
| IC50 | 0.2 uM | Not Available | BindingDB 17638 |
| IC50 | 0.25 uM | Not Available | BindingDB 17638 |
| IC50 | 0.26 uM | Not Available | BindingDB 17638 |
| IC50 | 0.5 uM | Not Available | BindingDB 17638 |
References
- Higuchi K, Tominaga K, Watanabe T, Uno H, Shiba M, Sasaki E, Tanigawa T, Takashima T, Hamaguchi M, Oshitani N, Matsumoto T, Iwanaga Y, Fukuda T, Fujiwara Y, Arakawa T: Indomethacin, but not Helicobacter pylori, inhibits adaptive relaxation in isolated guinea-pig stomach. Drugs Exp Clin Res. 2004;30(5-6):235-41. [15700751 ]
- Bobadilla L RA, Perez-Alvarez V, Bracho Valdes I, Lopez-Sanchez P: Effect of pregnancy on the roles of nitric oxide and prostaglandins in 5-hydroxytryptamine-induced contractions in rat isolated thoracic and abdominal aorta. Clin Exp Pharmacol Physiol. 2005 Mar;32(3):202-9. [15743404 ]
- Fornai M, Blandizzi C, Colucci R, Antonioli L, Bernardini N, Segnani C, Baragatti B, Barogi S, Berti P, Spisni R, Del Tacca M: Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut. 2005 May;54(5):608-16. [15831902 ]
- Moth CW, Prusakiewicz JJ, Marnett LJ, Lybrand TP: Stereoselective binding of indomethacin ethanolamide derivatives to cyclooxygenase-1. J Med Chem. 2005 May 19;48(10):3613-20. [15887968 ]
- Kundu N, Walser TC, Ma X, Fulton AM: Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol Immunother. 2005 Oct;54(10):981-7. Epub 2005 May 13. [15891886 ]
- Larsson J, Gottfries J, Bohlin L, Backlund A: Expanding the ChemGPS chemical space with natural products. J Nat Prod. 2005 Jul;68(7):985-91. [16038536 ]
- Prasit P, Wang Z, Brideau C, Chan CC, Charleson S, Cromlish W, Ethier D, Evans JF, Ford-Hutchinson AW, Gauthier JY, Gordon R, Guay J, Gresser M, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini J, O'Neill GP, Ouellet M, Percival MD, Perrier H, Riendeau D, Rodger I, Zamboni R, et al.: The discovery of rofecoxib, [MK 966, Vioxx, 4-(4'-methylsulfonylphenyl)-3-phenyl-2(5H)-furanone], an orally active cyclooxygenase-2-inhibitor. Bioorg Med Chem Lett. 1999 Jul 5;9(13):1773-8. [10406640 ]
- Liedtke AJ, Adeniji AO, Chen M, Byrns MC, Jin Y, Christianson DW, Marnett LJ, Penning TM: Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem. 2013 Mar 28;56(6):2429-46. doi: 10.1021/jm3017656. Epub 2013 Mar 13. [23432095 ]
- Chen XY, Park SJ, Buschmann H, De Rosa M, Bolm C: Syntheses and biological activities of sulfoximine-based acyclic triaryl olefins. Bioorg Med Chem Lett. 2012 Jul 1;22(13):4307-9. doi: 10.1016/j.bmcl.2012.05.018. Epub 2012 May 11. [22652053 ]
- Khan KM, Ambreen N, Mughal UR, Jalil S, Perveen S, Choudhary MI: 3-Formylchromones: potential antiinflammatory agents. Eur J Med Chem. 2010 Sep;45(9):4058-64. doi: 10.1016/j.ejmech.2010.05.065. Epub 2010 Jun 8. [20576329 ]
- Liedtke AJ, Crews BC, Daniel CM, Blobaum AL, Kingsley PJ, Ghebreselasie K, Marnett LJ: Cyclooxygenase-1-selective inhibitors based on the (E)-2'-des-methyl-sulindac sulfide scaffold. J Med Chem. 2012 Mar 8;55(5):2287-300. doi: 10.1021/jm201528b. Epub 2012 Feb 14. [22263894 ]
- Kakuta H, Zheng X, Oda H, Harada S, Sugimoto Y, Sasaki K, Tai A: Cyclooxygenase-1-selective inhibitors are attractive candidates for analgesics that do not cause gastric damage. design and in vitro/in vivo evaluation of a benzamide-type cyclooxygenase-1 selective inhibitor. J Med Chem. 2008 Apr 24;51(8):2400-11. doi: 10.1021/jm701191z. Epub 2008 Mar 26. [18363350 ]
- Hashimoto H, Imamura K, Haruta J, Wakitani K: 4-(4-cycloalkyl/aryl-oxazol-5-yl)benzenesulfonamides as selective cyclooxygenase-2 inhibitors: enhancement of the selectivity by introduction of a fluorine atom and identification of a potent, highly selective, and orally active COX-2 inhibitor JTE-522(1). J Med Chem. 2002 Mar 28;45(7):1511-7. [11906292 ]
- Bridoux A, Millet R, Pommery J, Pommery N, Henichart JP: Synthesis and biological activity of N-aroyl-tetrahydro-gamma-carbolines. Bioorg Med Chem. 2010 Jun 1;18(11):3910-24. doi: 10.1016/j.bmc.2010.04.034. Epub 2010 Apr 18. [20451397 ]
- Puig C, Crespo MI, Godessart N, Feixas J, Ibarzo J, Jimenez JM, Soca L, Cardelus I, Heredia A, Miralpeix M, Puig J, Beleta J, Huerta JM, Lopez M, Segarra V, Ryder H, Palacios JM: Synthesis and biological evaluation of 3,4-diaryloxazolones: A new class of orally active cyclooxygenase-2 inhibitors. J Med Chem. 2000 Jan 27;43(2):214-23. [10649977 ]
- Bekhit AA, Ashour HM, Bekhit Ael-D, Abdel-Rahman HM, Bekhit SA: Synthesis of some pyrazolyl benzenesulfonamide derivatives as dual anti-inflammatory antimicrobial agents. J Enzyme Inhib Med Chem. 2009 Feb;24(1):296-309. doi: 10.1080/14756360802188404 . [18951238 ]
- Wey SJ, Augustyniak ME, Cochran ED, Ellis JL, Fang X, Garvey DS, Janero DR, Letts LG, Martino AM, Melim TL, Murty MG, Richardson SK, Schroeder JD, Selig WM, Trocha AM, Wexler RS, Young DV, Zemtseva IS, Zifcak BM: Structure-based design, synthesis, and biological evaluation of indomethacin derivatives as cyclooxygenase-2 inhibiting nitric oxide donors. J Med Chem. 2007 Dec 13;50(25):6367-82. Epub 2007 Nov 10. [17994684 ]
- Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Falgueyret JP, Ford-Hutchinson AW, Gordon R, Greig G, Gresser M, Guay J, Kargman S, Leger S, Mancini JA, O'Neill G, Ouellet M, Rodger IW, Therien M, Wang Z, Webb JK, Wong E, Chan CC, et al.: Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997 May;121(1):105-17. [9146894 ]
- General Function:
- Trans-1,2-dihydrobenzene-1,2-diol dehydrogenase activity
- Specific Function:
- Catalyzes the conversion of aldehydes and ketones to alcohols. Catalyzes the reduction of prostaglandin (PG) D2, PGH2 and phenanthrenequinone (PQ) and the oxidation of 9-alpha,11-beta-PGF2 to PGD2. Functions as a bi-directional 3-alpha-, 17-beta- and 20-alpha HSD. Can interconvert active androgens, estrogens and progestins with their cognate inactive metabolites. Preferentially transforms androstenedione (4-dione) to testosterone.
- Gene Name:
- AKR1C3
- Uniprot ID:
- P42330
- Molecular Weight:
- 36852.89 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.1 uM | Not Available | BindingDB 17638 |
| IC50 | 0.73 uM | Not Available | BindingDB 17638 |
| IC50 | 2.3 uM | Not Available | BindingDB 17638 |
| IC50 | 4.1 uM | Not Available | BindingDB 17638 |
References
- Liedtke AJ, Adeniji AO, Chen M, Byrns MC, Jin Y, Christianson DW, Marnett LJ, Penning TM: Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem. 2013 Mar 28;56(6):2429-46. doi: 10.1021/jm3017656. Epub 2013 Mar 13. [23432095 ]
- Jamieson SM, Brooke DG, Heinrich D, Atwell GJ, Silva S, Hamilton EJ, Turnbull AP, Rigoreau LJ, Trivier E, Soudy C, Samlal SS, Owen PJ, Schroeder E, Raynham T, Flanagan JU, Denny WA: 3-(3,4-Dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic Acids: highly potent and selective inhibitors of the type 5 17-beta-hydroxysteroid dehydrogenase AKR1C3. J Med Chem. 2012 Sep 13;55(17):7746-58. doi: 10.1021/jm3007867. Epub 2012 Aug 21. [22877157 ]
- Endo S, Matsunaga T, Kanamori A, Otsuji Y, Nagai H, Sundaram K, El-Kabbani O, Toyooka N, Ohta S, Hara A: Selective inhibition of human type-5 17beta-hydroxysteroid dehydrogenase (AKR1C3) by baccharin, a component of Brazilian propolis. J Nat Prod. 2012 Apr 27;75(4):716-21. doi: 10.1021/np201002x. Epub 2012 Apr 16. [22506594 ]
- Lovering AL, Ride JP, Bunce CM, Desmond JC, Cummings SM, White SA: Crystal structures of prostaglandin D(2) 11-ketoreductase (AKR1C3) in complex with the nonsteroidal anti-inflammatory drugs flufenamic acid and indomethacin. Cancer Res. 2004 Mar 1;64(5):1802-10. [14996743 ]
- General Function:
- Prostaglandin j receptor activity
- Specific Function:
- Receptor for prostaglandin D2 (PGD2). Coupled to the G(i)-protein. Receptor activation may result in pertussis toxin-sensitive decreases in cAMP levels and Ca(2+) mobilization. PI3K signaling is also implicated in mediating PTGDR2 effects. PGD2 induced receptor internalization. CRTH2 internalization can be regulated by diverse kinases such as, PKC, PKA, ADRBK1/GRK2, GPRK5/GRK5 and GRK6. Receptor activation is responsible, at least in part, in immune regulation and allergic/inflammation responses.
- Gene Name:
- PTGDR2
- Uniprot ID:
- Q9Y5Y4
- Molecular Weight:
- 43267.15 Da
References
- Hata AN, Lybrand TP, Breyer RM: Identification of determinants of ligand binding affinity and selectivity in the prostaglandin D2 receptor CRTH2. J Biol Chem. 2005 Sep 16;280(37):32442-51. Epub 2005 Jul 19. [16030019 ]
- Hata AN, Lybrand TP, Marnett LJ, Breyer RM: Structural determinants of arylacetic acid nonsteroidal anti-inflammatory drugs necessary for binding and activation of the prostaglandin D2 receptor CRTH2. Mol Pharmacol. 2005 Mar;67(3):640-7. Epub 2004 Nov 24. [15563582 ]
- Mathiesen JM, Ulven T, Martini L, Gerlach LO, Heinemann A, Kostenis E: Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol Pharmacol. 2005 Aug;68(2):393-402. Epub 2005 May 3. [15870392 ]
- Sugimoto H, Shichijo M, Okano M, Bacon KB: CRTH2-specific binding characteristics of [3H]ramatroban and its effects on PGD2-, 15-deoxy-Delta12, 14-PGJ2- and indomethacin-induced agonist responses. Eur J Pharmacol. 2005 Nov 7;524(1-3):30-7. Epub 2005 Oct 27. [16256979 ]
- General Function:
- Trans-1,2-dihydrobenzene-1,2-diol dehydrogenase activity
- Specific Function:
- Works in concert with the 5-alpha/5-beta-steroid reductases to convert steroid hormones into the 3-alpha/5-alpha and 3-alpha/5-beta-tetrahydrosteroids. Catalyzes the inactivation of the most potent androgen 5-alpha-dihydrotestosterone (5-alpha-DHT) to 5-alpha-androstane-3-alpha,17-beta-diol (3-alpha-diol). Has a high bile-binding ability.
- Gene Name:
- AKR1C2
- Uniprot ID:
- P52895
- Molecular Weight:
- 36734.97 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 36.53 uM | Not Available | BindingDB 17638 |
| IC50 | 75 uM | Not Available | BindingDB 17638 |
| IC50 | >100 uM | Not Available | BindingDB 17638 |
References
- Liedtke AJ, Adeniji AO, Chen M, Byrns MC, Jin Y, Christianson DW, Marnett LJ, Penning TM: Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem. 2013 Mar 28;56(6):2429-46. doi: 10.1021/jm3017656. Epub 2013 Mar 13. [23432095 ]
- Endo S, Matsunaga T, Kanamori A, Otsuji Y, Nagai H, Sundaram K, El-Kabbani O, Toyooka N, Ohta S, Hara A: Selective inhibition of human type-5 17beta-hydroxysteroid dehydrogenase (AKR1C3) by baccharin, a component of Brazilian propolis. J Nat Prod. 2012 Apr 27;75(4):716-21. doi: 10.1021/np201002x. Epub 2012 Apr 16. [22506594 ]
- Jamieson SM, Brooke DG, Heinrich D, Atwell GJ, Silva S, Hamilton EJ, Turnbull AP, Rigoreau LJ, Trivier E, Soudy C, Samlal SS, Owen PJ, Schroeder E, Raynham T, Flanagan JU, Denny WA: 3-(3,4-Dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic Acids: highly potent and selective inhibitors of the type 5 17-beta-hydroxysteroid dehydrogenase AKR1C3. J Med Chem. 2012 Sep 13;55(17):7746-58. doi: 10.1021/jm3007867. Epub 2012 Aug 21. [22877157 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Catalyzes the conversion of hemimercaptal, formed from methylglyoxal and glutathione, to S-lactoylglutathione. Involved in the regulation of TNF-induced transcriptional activity of NF-kappa-B. Required for normal osteoclastogenesis.
- Gene Name:
- GLO1
- Uniprot ID:
- Q04760
- Molecular Weight:
- 20777.515 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 18.1 uM | Not Available | BindingDB 17638 |
| Inhibitory | 24 uM | Not Available | BindingDB 17638 |
| Inhibitory | 24.4 uM | Not Available | BindingDB 17638 |
References
- Sato S, Kwon Y, Kamisuki S, Srivastava N, Mao Q, Kawazoe Y, Uesugi M: Polyproline-rod approach to isolating protein targets of bioactive small molecules: isolation of a new target of indomethacin. J Am Chem Soc. 2007 Jan 31;129(4):873-80. [17243824 ]
- Yuan M, Luo M, Song Y, Xu Q, Wang X, Cao Y, Bu X, Ren Y, Hu X: Identification of curcumin derivatives as human glyoxalase I inhibitors: A combination of biological evaluation, molecular docking, 3D-QSAR and molecular dynamics simulation studies. Bioorg Med Chem. 2011 Feb 1;19(3):1189-96. doi: 10.1016/j.bmc.2010.12.039. Epub 2010 Dec 22. [21237663 ]
- Liu M, Yuan M, Li Z, Cheng YK, Luo HB, Hu X: Structural investigation into the inhibitory mechanisms of indomethacin and its analogues towards human glyoxalase I. Bioorg Med Chem Lett. 2011 Jul 15;21(14):4243-7. doi: 10.1016/j.bmcl.2011.05.095. Epub 2011 Jun 1. [21689932 ]
- General Function:
- Trans-1,2-dihydrobenzene-1,2-diol dehydrogenase activity
- Specific Function:
- Converts progesterone to its inactive form, 20-alpha-dihydroxyprogesterone (20-alpha-OHP). In the liver and intestine, may have a role in the transport of bile. May have a role in monitoring the intrahepatic bile acid concentration. Has a low bile-binding ability. May play a role in myelin formation.
- Gene Name:
- AKR1C1
- Uniprot ID:
- Q04828
- Molecular Weight:
- 36788.02 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 130 uM | Not Available | BindingDB 17638 |
| IC50 | >100 uM | Not Available | BindingDB 17638 |
References
- Endo S, Matsunaga T, Kanamori A, Otsuji Y, Nagai H, Sundaram K, El-Kabbani O, Toyooka N, Ohta S, Hara A: Selective inhibition of human type-5 17beta-hydroxysteroid dehydrogenase (AKR1C3) by baccharin, a component of Brazilian propolis. J Nat Prod. 2012 Apr 27;75(4):716-21. doi: 10.1021/np201002x. Epub 2012 Apr 16. [22506594 ]
- Jamieson SM, Brooke DG, Heinrich D, Atwell GJ, Silva S, Hamilton EJ, Turnbull AP, Rigoreau LJ, Trivier E, Soudy C, Samlal SS, Owen PJ, Schroeder E, Raynham T, Flanagan JU, Denny WA: 3-(3,4-Dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic Acids: highly potent and selective inhibitors of the type 5 17-beta-hydroxysteroid dehydrogenase AKR1C3. J Med Chem. 2012 Sep 13;55(17):7746-58. doi: 10.1021/jm3007867. Epub 2012 Aug 21. [22877157 ]
- General Function:
- Retinal dehydrogenase activity
- Specific Function:
- Catalyzes the transformation of the potent androgen dihydrotestosterone (DHT) into the less active form, 5-alpha-androstan-3-alpha,17-beta-diol (3-alpha-diol). Also has some 20-alpha-hydroxysteroid dehydrogenase activity. The biotransformation of the pesticide chlordecone (kepone) to its corresponding alcohol leads to increased biliary excretion of the pesticide and concomitant reduction of its neurotoxicity since bile is the major excretory route.
- Gene Name:
- AKR1C4
- Uniprot ID:
- P17516
- Molecular Weight:
- 37066.52 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 54 uM | Not Available | BindingDB 17638 |
| IC50 | >100 uM | Not Available | BindingDB 17638 |
References
- Endo S, Matsunaga T, Kanamori A, Otsuji Y, Nagai H, Sundaram K, El-Kabbani O, Toyooka N, Ohta S, Hara A: Selective inhibition of human type-5 17beta-hydroxysteroid dehydrogenase (AKR1C3) by baccharin, a component of Brazilian propolis. J Nat Prod. 2012 Apr 27;75(4):716-21. doi: 10.1021/np201002x. Epub 2012 Apr 16. [22506594 ]
- Jamieson SM, Brooke DG, Heinrich D, Atwell GJ, Silva S, Hamilton EJ, Turnbull AP, Rigoreau LJ, Trivier E, Soudy C, Samlal SS, Owen PJ, Schroeder E, Raynham T, Flanagan JU, Denny WA: 3-(3,4-Dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic Acids: highly potent and selective inhibitors of the type 5 17-beta-hydroxysteroid dehydrogenase AKR1C3. J Med Chem. 2012 Sep 13;55(17):7746-58. doi: 10.1021/jm3007867. Epub 2012 Aug 21. [22877157 ]
- General Function:
- Prostaglandin e receptor activity
- Specific Function:
- Receptor for prostaglandin E2 (PGE2). The activity of this receptor is mediated by G(s) proteins that stimulate adenylate cyclase. The subsequent raise in intracellular cAMP is responsible for the relaxing effect of this receptor on smooth muscle.
- Gene Name:
- PTGER2
- Uniprot ID:
- P43116
- Molecular Weight:
- 39759.945 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.007 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.018 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.0198 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.021 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.027 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.057 uM | Not Available | BindingDB 17638 |
| Inhibitory | 0.1 uM | Not Available | BindingDB 17638 |
References
- Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Ford-Hutchinson AW, Forrest MJ, Gauthier JY, Gordon R, Gresser M, Guay J, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini J, O'Neill GP, Ouellet M, Patrick D, Percival MD, Perrier H, Prasit P, Rodger I, et al.: Rofecoxib [Vioxx, MK-0966; 4-(4'-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999 Aug;290(2):551-60. [10411562 ]
- Riendeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret JP, Friesen RW, Gordon R, Greig G, Guay J, Mancini J, Ouellet M, Wong E, Xu L, Boyce S, Visco D, Girard Y, Prasit P, Zamboni R, Rodger IW, Gresser M, Ford-Hutchinson AW, Young RN, Chan CC: Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther. 2001 Feb;296(2):558-66. [11160644 ]
- General Function:
- Sodium-independent organic anion transmembrane transporter activity
- Specific Function:
- Mediates the Na(+)-independent uptake of organic anions such as pravastatin, taurocholate, methotrexate, dehydroepiandrosterone sulfate, 17-beta-glucuronosyl estradiol, estrone sulfate, prostaglandin E2, thromboxane B2, leukotriene C3, leukotriene E4, thyroxine and triiodothyronine. Involved in the clearance of bile acids and organic anions from the liver.
- Gene Name:
- SLCO1B1
- Uniprot ID:
- Q9Y6L6
- Molecular Weight:
- 76447.99 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.002 uM | Not Available | BindingDB 17638 |
| Inhibitory | 11 uM | Not Available | BindingDB 17638 |
| IC50 | 12 uM | Not Available | BindingDB 17638 |
References
- Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, Haglund U, Artursson P: Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem. 2012 May 24;55(10):4740-63. doi: 10.1021/jm300212s. Epub 2012 May 15. [22541068 ]
- Riendeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret JP, Friesen RW, Gordon R, Greig G, Guay J, Mancini J, Ouellet M, Wong E, Xu L, Boyce S, Visco D, Girard Y, Prasit P, Zamboni R, Rodger IW, Gresser M, Ford-Hutchinson AW, Young RN, Chan CC: Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther. 2001 Feb;296(2):558-66. [11160644 ]
- General Function:
- Glyceraldehyde oxidoreductase activity
- Specific Function:
- Catalyzes the NADPH-dependent reduction of a wide variety of carbonyl-containing compounds to their corresponding alcohols with a broad range of catalytic efficiencies.
- Gene Name:
- AKR1B1
- Uniprot ID:
- P15121
- Molecular Weight:
- 35853.125 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 6 uM | Not Available | BindingDB 17638 |
References
- Cerelli MJ, Curtis DL, Dunn JP, Nelson PH, Peak TM, Waterbury LD: Antiinflammatory and aldose reductase inhibitory activity of some tricyclic arylacetic acids. J Med Chem. 1986 Nov;29(11):2347-51. [3097317 ]
- General Function:
- Iron ion binding
- Specific Function:
- Catalyzes the first step in leukotriene biosynthesis, and thereby plays a role in inflammatory processes.
- Gene Name:
- ALOX5
- Uniprot ID:
- P09917
- Molecular Weight:
- 77982.595 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 7 uM | Not Available | BindingDB 17638 |
References
- Kolasa T, Brooks CD, Rodriques KE, Summers JB, Dellaria JF, Hulkower KI, Bouska J, Bell RL, Carter GW: Nonsteroidal anti-inflammatory drugs as scaffolds for the design of 5-lipoxygenase inhibitors. J Med Chem. 1997 Feb 28;40(5):819-24. [9057869 ]
- General Function:
- Toxic substance binding
- Specific Function:
- Bifunctional enzyme. The C-terminal domain has epoxide hydrolase activity and acts on epoxides (alkene oxides, oxiranes) and arene oxides. Plays a role in xenobiotic metabolism by degrading potentially toxic epoxides. Also determines steady-state levels of physiological mediators. The N-terminal domain has lipid phosphatase activity, with the highest activity towards threo-9,10-phosphonooxy-hydroxy-octadecanoic acid, followed by erythro-9,10-phosphonooxy-hydroxy-octadecanoic acid, 12-phosphonooxy-octadec-9Z-enoic acid, 12-phosphonooxy-octadec-9E-enoic acid, and p-nitrophenyl phospate.
- Gene Name:
- EPHX2
- Uniprot ID:
- P34913
- Molecular Weight:
- 62615.22 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >1 uM | Not Available | BindingDB 17638 |
References
- Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD: Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem. 2011 Apr 28;54(8):3037-50. doi: 10.1021/jm2001376. Epub 2011 Apr 5. [21434686 ]
- General Function:
- Organic anion transmembrane transporter activity
- Specific Function:
- Mediates hepatobiliary excretion of numerous organic anions. May function as a cellular cisplatin transporter.
- Gene Name:
- ABCC2
- Uniprot ID:
- Q92887
- Molecular Weight:
- 174205.64 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 235 uM | Not Available | BindingDB 17638 |
References
- Leyers S, Hacker HG, Wiendlocha J, Gutschow M, Wiese M: A 4-aminobenzoic acid derivative as novel lead for selective inhibitors of multidrug resistance-associated proteins. Bioorg Med Chem Lett. 2008 Sep 1;18(17):4761-3. doi: 10.1016/j.bmcl.2008.07.127. Epub 2008 Aug 3. [18707884 ]
- General Function:
- Testosterone dehydrogenase (nad+) activity
- Specific Function:
- 3-alpha-hydroxysteroid dehydrogenase that converts 3-alpha-tetrahydroprogesterone (allopregnanolone) to dihydroxyprogesterone and 3-alpha-androstanediol to dihydroxyprogesterone. May play a role in the biosynthesis of retinoic acid from retinaldehyde, but seems to have low activity with retinoids. Can utilize both NADH and NADPH.
- Gene Name:
- DHRS9
- Uniprot ID:
- Q9BPW9
- Molecular Weight:
- 35226.78 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 14 uM | Not Available | BindingDB 17638 |
References
- Gebhardt P, Dornberger K, Gollmick FA, Grafe U, Hartl A, Gorls H, Schlegel B, Hertweck C: Quercinol, an anti-inflammatory chromene from the wood-rotting fungus Daedalea quercina (Oak Mazegill). Bioorg Med Chem Lett. 2007 May 1;17(9):2558-60. Epub 2007 Feb 7. [17346963 ]
- General Function:
- Interleukin-8 receptor binding
- Specific Function:
- IL-8 is a chemotactic factor that attracts neutrophils, basophils, and T-cells, but not monocytes. It is also involved in neutrophil activation. It is released from several cell types in response to an inflammatory stimulus. IL-8(6-77) has a 5-10-fold higher activity on neutrophil activation, IL-8(5-77) has increased activity on neutrophil activation and IL-8(7-77) has a higher affinity to receptors CXCR1 and CXCR2 as compared to IL-8(1-77), respectively.
- Gene Name:
- CXCL8
- Uniprot ID:
- P10145
- Molecular Weight:
- 11097.98 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.05 uM | Not Available | BindingDB 17638 |
References
- Sablone MR, Cesta MC, Moriconi A, Aramini A, Bizzarri C, Di Giacinto C, Di Bitondo R, Gloaguen I, Aschi M, Crucianelli M, Bertini R, Allegretti M: Structure-Activity Relationship of novel phenylacetic CXCR1 inhibitors. Bioorg Med Chem Lett. 2009 Aug 1;19(15):4026-30. doi: 10.1016/j.bmcl.2009.06.027. Epub 2009 Jun 13. [19560921 ]
- General Function:
- Monovalent cation:proton antiporter activity
- Specific Function:
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide (NMN), metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfate, acyclovir, ganciclovir and also the zwitterionic cephalosporin, cephalexin and cephradin. Seems to also play a role in the uptake of oxaliplatin (a new platinum anticancer agent). Able to transport paraquat (PQ or N,N-dimethyl-4-4'-bipiridinium); a widely used herbicid. Responsible for the secretion of cationic drugs across the brush border membranes.
- Gene Name:
- SLC47A1
- Uniprot ID:
- Q96FL8
- Molecular Weight:
- 61921.585 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >500 uM | Not Available | BindingDB 17638 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- General Function:
- Transporter activity
- Specific Function:
- Mediates export of organic anions and drugs from the cytoplasm. Mediates ATP-dependent transport of glutathione and glutathione conjugates, leukotriene C4, estradiol-17-beta-o-glucuronide, methotrexate, antiviral drugs and other xenobiotics. Confers resistance to anticancer drugs. Hydrolyzes ATP with low efficiency.
- Gene Name:
- ABCC1
- Uniprot ID:
- P33527
- Molecular Weight:
- 171589.5 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 12 uM | Not Available | BindingDB 17638 |
References
- Leyers S, Hacker HG, Wiendlocha J, Gutschow M, Wiese M: A 4-aminobenzoic acid derivative as novel lead for selective inhibitors of multidrug resistance-associated proteins. Bioorg Med Chem Lett. 2008 Sep 1;18(17):4761-3. doi: 10.1016/j.bmcl.2008.07.127. Epub 2008 Aug 3. [18707884 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Ligand-activated transcription factor. Key regulator of lipid metabolism. Activated by the endogenous ligand 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC). Activated by oleylethanolamide, a naturally occurring lipid that regulates satiety. Receptor for peroxisome proliferators such as hypolipidemic drugs and fatty acids. Regulates the peroxisomal beta-oxidation pathway of fatty acids. Functions as transcription activator for the ACOX1 and P450 genes. Transactivation activity requires heterodimerization with RXRA and is antagonized by NR2C2. May be required for the propagation of clock information to metabolic pathways regulated by PER2.
- Gene Name:
- PPARA
- Uniprot ID:
- Q07869
- Molecular Weight:
- 52224.595 Da
References
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA: Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997 Feb 7;272(6):3406-10. [9013583 ]
- General Function:
- Prostaglandin-e synthase activity
- Specific Function:
- Catalyzes the oxidoreduction of prostaglandin endoperoxide H2 (PGH2) to prostaglandin E2 (PGE2).
- Gene Name:
- PTGES
- Uniprot ID:
- O14684
- Molecular Weight:
- 17102.135 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 40.6 uM | Not Available | BindingDB 17638 |
References
- Elkady M, Niess R, Schaible AM, Bauer J, Luderer S, Ambrosi G, Werz O, Laufer SA: Modified acidic nonsteroidal anti-inflammatory drugs as dual inhibitors of mPGES-1 and 5-LOX. J Med Chem. 2012 Oct 25;55(20):8958-62. doi: 10.1021/jm3010543. Epub 2012 Oct 9. [22992107 ]
- General Function:
- 15-oxoprostaglandin 13-oxidase activity
- Specific Function:
- Functions as 15-oxo-prostaglandin 13-reductase and acts on 15-keto-PGE1, 15-keto-PGE2, 15-keto-PGE1-alpha and 15-keto-PGE2-alpha with highest activity towards 15-keto-PGE2. Overexpression represses transcriptional activity of PPARG and inhibits adipocyte differentiation (By similarity).
- Gene Name:
- PTGR2
- Uniprot ID:
- Q8N8N7
- Molecular Weight:
- 38498.74 Da
References
- Wu YH, Ko TP, Guo RT, Hu SM, Chuang LM, Wang AH: Structural basis for catalytic and inhibitory mechanisms of human prostaglandin reductase PTGR2. Structure. 2008 Nov 12;16(11):1714-23. doi: 10.1016/j.str.2008.09.007. [19000823 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Hydrolyzes the toxic metabolites of a variety of organophosphorus insecticides. Capable of hydrolyzing a broad spectrum of organophosphate substrates and lactones, and a number of aromatic carboxylic acid esters. Mediates an enzymatic protection of low density lipoproteins against oxidative modification and the consequent series of events leading to atheroma formation.
- Gene Name:
- PON1
- Uniprot ID:
- P27169
- Molecular Weight:
- 39730.99 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 97 uM | Not Available | BindingDB 17638 |
| IC50 | 195 uM | Not Available | BindingDB 17638 |
References
- Ekinci D, Beydemir S: Effect of some analgesics on paraoxonase-1 purified from human serum. J Enzyme Inhib Med Chem. 2009 Aug;24(4):1034-9. doi: 10.1080/14756360802608351. [19548782 ]
- General Function:
- Sodium-independent organic anion transmembrane transporter activity
- Specific Function:
- Involved in the renal elimination of endogenous and exogenous organic anions. Functions as organic anion exchanger when the uptake of one molecule of organic anion is coupled with an efflux of one molecule of endogenous dicarboxylic acid (glutarate, ketoglutarate, etc). Mediates the sodium-independent uptake of 2,3-dimercapto-1-propanesulfonic acid (DMPS) (By similarity). Mediates the sodium-independent uptake of p-aminohippurate (PAH), ochratoxin (OTA), acyclovir (ACV), 3'-azido-3-'deoxythymidine (AZT), cimetidine (CMD), 2,4-dichloro-phenoxyacetate (2,4-D), hippurate (HA), indoleacetate (IA), indoxyl sulfate (IS) and 3-carboxy-4-methyl-5-propyl-2-furanpropionate (CMPF), cidofovir, adefovir, 9-(2-phosphonylmethoxyethyl) guanine (PMEG), 9-(2-phosphonylmethoxyethyl) diaminopurine (PMEDAP) and edaravone sulfate. PAH uptake is inhibited by p-chloromercuribenzenesulphonate (PCMBS), diethyl pyrocarbonate (DEPC), sulindac, diclofenac, carprofen, glutarate and okadaic acid (By similarity). PAH uptake is inhibited by benzothiazolylcysteine (BTC), S-chlorotrifluoroethylcysteine (CTFC), cysteine S-conjugates S-dichlorovinylcysteine (DCVC), furosemide, steviol, phorbol 12-myristate 13-acetate (PMA), calcium ionophore A23187, benzylpenicillin, furosemide, indomethacin, bumetamide, losartan, probenecid, phenol red, urate, and alpha-ketoglutarate.
- Gene Name:
- SLC22A6
- Uniprot ID:
- Q4U2R8
- Molecular Weight:
- 61815.78 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 3 uM | Not Available | BindingDB 17638 |
References
- Mulato AS, Ho ES, Cihlar T: Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther. 2000 Oct;295(1):10-5. [10991954 ]
- General Function:
- Sodium-independent organic anion transmembrane transporter activity
- Specific Function:
- Plays an important role in the excretion/detoxification of endogenous and exogenous organic anions, especially from the brain and kidney. Involved in the transport basolateral of steviol, fexofenadine. Transports benzylpenicillin (PCG), estrone-3-sulfate (E1S), cimetidine (CMD), 2,4-dichloro-phenoxyacetate (2,4-D), p-amino-hippurate (PAH), acyclovir (ACV) and ochratoxin (OTA).
- Gene Name:
- SLC22A8
- Uniprot ID:
- Q8TCC7
- Molecular Weight:
- 59855.585 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 5.95 uM | Not Available | BindingDB 17638 |
References
- Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, Sekine T, Endou H: Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002 Aug;302(2):666-71. [12130730 ]
- General Function:
- Sodium-independent organic anion transmembrane transporter activity
- Specific Function:
- Mediates the Na(+)-independent uptake of organic anions such as 17-beta-glucuronosyl estradiol, taurocholate, triiodothyronine (T3), leukotriene C4, dehydroepiandrosterone sulfate (DHEAS), methotrexate and sulfobromophthalein (BSP). Involved in the clearance of bile acids and organic anions from the liver.
- Gene Name:
- SLCO1B3
- Uniprot ID:
- Q9NPD5
- Molecular Weight:
- 77402.175 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 38 uM | Not Available | BindingDB 17638 |
| IC50 | 41 uM | Not Available | BindingDB 17638 |
References
- Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, Haglund U, Artursson P: Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem. 2012 May 24;55(10):4740-63. doi: 10.1021/jm300212s. Epub 2012 May 15. [22541068 ]
- General Function:
- Sodium-independent organic anion transmembrane transporter activity
- Specific Function:
- Mediates the Na(+)-independent transport of organic anions such as taurocholate, the prostaglandins PGD2, PGE1, PGE2, leukotriene C4, thromboxane B2 and iloprost.
- Gene Name:
- SLCO2B1
- Uniprot ID:
- O94956
- Molecular Weight:
- 76709.98 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 130 uM | Not Available | BindingDB 17638 |
| IC50 | 140 uM | Not Available | BindingDB 17638 |
References
- Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, Haglund U, Artursson P: Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J Med Chem. 2012 May 24;55(10):4740-63. doi: 10.1021/jm300212s. Epub 2012 May 15. [22541068 ]
- General Function:
- Monoamine transmembrane transporter activity
- Specific Function:
- Amine transporter. Terminates the action of dopamine by its high affinity sodium-dependent reuptake into presynaptic terminals.
- Gene Name:
- SLC6A3
- Uniprot ID:
- Q01959
- Molecular Weight:
- 68494.255 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| AC50 | 4.95 uM | NVS_TR_hDAT | Novascreen |
References
- Sipes NS, Martin MT, Kothiya P, Reif DM, Judson RS, Richard AM, Houck KA, Dix DJ, Kavlock RJ, Knudsen TB: Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem Res Toxicol. 2013 Jun 17;26(6):878-95. doi: 10.1021/tx400021f. Epub 2013 May 16. [23611293 ]
- General Function:
- Phospholipid binding
- Specific Function:
- Thought to participate in the regulation of the phospholipid metabolism in biomembranes including eicosanoid biosynthesis. Catalyzes the calcium-dependent hydrolysis of the 2-acyl groups in 3-sn-phosphoglycerides.
- Gene Name:
- PLA2G2A
- Uniprot ID:
- P14555
- Molecular Weight:
- 16082.525 Da