Toxaphene (T3D0031)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-03-06 18:57:57 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:20:55 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D0031 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Toxaphene | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
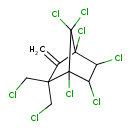
| Description | Toxaphene is an insecticide mixture of compounds. It was once widenly used, but it is now banned in most areas due to its toxicity and tendency to bioaccumulate in the environment. (5) The structure displayed is a generic structure of toxaphene components. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C10H8Cl8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 411.795 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 407.813 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 8001-35-2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 1,4,5,6,7,7-hexachloro-2,2-bis(chloromethyl)-3-methylidenebicyclo[2.2.1]heptane | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | toxaphene | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | ClCC1(CCl)C(=C)C2(Cl)C(Cl)C(Cl)C1(Cl)C2(Cl)Cl | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C10H8Cl8/c1-4-7(2-11,3-12)9(16)6(14)5(13)8(4,15)10(9,17)18/h5-6H,1-3H2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=OEJNXTAZZBRGDN-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as bicyclic monoterpenoids. These are monoterpenoids containing exactly 2 rings, which are fused to each other. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Monoterpenoids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Bicyclic monoterpenoids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic homopolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Yellow solid. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral (5) ; inhalation (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Toxaphene is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Toxaphene is absorbed through the intestines and lungs, then distributed mainly to fat tissues. Due to the complex composition of toxaphene, it requires various metabolic pathways to degrade, which involve dechlorination, dehydrodechlorination, and oxidation. Metabolism occurs primarily by hepatic mixed-function oxidases, and metabolites are excreted in the urine and faeces. (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 50 mg/kg (Oral, Rat) (8) LD50: 600 mg/kg (Dermal, Rat) (8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | 2 to 7 grams for an adult human. (183) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Toxaphene was used as an insecticide. (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Acute Oral: 0.005 mg/kg/day (6) Intermediate Oral: 0.001 mg/kg/day (6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen. When there is an accumulation of ACh at autonomic ganglia this causes overstimulation of nicotinic expression in the sympathetic system. Symptoms associated with this are hypertension, and hypoglycemia. Overstimulation of nicotinic acetylcholine receptors in the central nervous system, due to accumulation of ACh, results in anxiety, headache, convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, and potentially coma. When there is expression of muscarinic overstimulation due to excess acetylcholine at muscarinic acetylcholine receptors symptoms of visual disturbances, tightness in chest, wheezing due to bronchoconstriction, increased bronchial secretions, increased salivation, lacrimation, sweating, peristalsis, and urination can occur. Certain reproductive effects in fertility, growth, and development for males and females have been linked specifically to organophosphate pesticide exposure. Most of the research on reproductive effects has been conducted on farmers working with pesticides and insecticdes in rural areas. In females menstrual cycle disturbances, longer pregnancies, spontaneous abortions, stillbirths, and some developmental effects in offspring have been linked to organophosphate pesticide exposure. Prenatal exposure has been linked to impaired fetal growth and development. Neurotoxic effects have also been linked to poisoning with OP pesticides causing four neurotoxic effects in humans: cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy (OPIDP), and chronic organophosphate-induced neuropsychiatric disorder (COPIND). These syndromes result after acute and chronic exposure to OP pesticides. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of low dose exposure include excessive salivation and eye-watering. Acute dose symptoms include severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Hypertension, hypoglycemia, anxiety, headache, tremor and ataxia may also result. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 5284469 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL1566504 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 4447533 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C15470 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | D014112 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Toxaphene | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | 1399 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D0031.pdf | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear hormone receptor. The steroid hormones and their receptors are involved in the regulation of eukaryotic gene expression and affect cellular proliferation and differentiation in target tissues. Ligand-dependent nuclear transactivation involves either direct homodimer binding to a palindromic estrogen response element (ERE) sequence or association with other DNA-binding transcription factors, such as AP-1/c-Jun, c-Fos, ATF-2, Sp1 and Sp3, to mediate ERE-independent signaling. Ligand binding induces a conformational change allowing subsequent or combinatorial association with multiprotein coactivator complexes through LXXLL motifs of their respective components. Mutual transrepression occurs between the estrogen receptor (ER) and NF-kappa-B in a cell-type specific manner. Decreases NF-kappa-B DNA-binding activity and inhibits NF-kappa-B-mediated transcription from the IL6 promoter and displace RELA/p65 and associated coregulators from the promoter. Recruited to the NF-kappa-B response element of the CCL2 and IL8 promoters and can displace CREBBP. Present with NF-kappa-B components RELA/p65 and NFKB1/p50 on ERE sequences. Can also act synergistically with NF-kappa-B to activate transcription involving respective recruitment adjacent response elements; the function involves CREBBP. Can activate the transcriptional activity of TFF1. Also mediates membrane-initiated estrogen signaling involving various kinase cascades. Isoform 3 is involved in activation of NOS3 and endothelial nitric oxide production. Isoforms lacking one or several functional domains are thought to modulate transcriptional activity by competitive ligand or DNA binding and/or heterodimerization with the full length receptor. Essential for MTA1-mediated transcriptional regulation of BRCA1 and BCAS3. Isoform 3 can bind to ERE and inhibit isoform 1.
- Gene Name:
- ESR1
- Uniprot ID:
- P03372
- Molecular Weight:
- 66215.45 Da
References
- Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M: N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006 Apr;69(8):1450-4. [16531984 ]
- Hodges LC, Bergerson JS, Hunter DS, Walker CL: Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 2000 Apr;54(2):355-64. [10774817 ]
- Chen S, Zhou D, Yang C, Sherman M: Molecular basis for the constitutive activity of estrogen-related receptor alpha-1. J Biol Chem. 2001 Jul 27;276(30):28465-70. Epub 2001 May 16. [11356845 ]
- Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R: Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006 Aug 15;79(12):1160-9. Epub 2006 Mar 27. [16626760 ]
- Luft S, Milki E, Glustrom E, Ampiah-Bonney R, O'Hara P. Binding of Organochloride and Pyrethroid Pesticides To Estrogen Receptors α and β: A Fluorescence Polarization Assay. Biophysical Journal 2009;96(3):444a.
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear hormone receptor. Binds estrogens with an affinity similar to that of ESR1, and activates expression of reporter genes containing estrogen response elements (ERE) in an estrogen-dependent manner (PubMed:20074560). Isoform beta-cx lacks ligand binding ability and has no or only very low ere binding activity resulting in the loss of ligand-dependent transactivation ability. DNA-binding by ESR1 and ESR2 is rapidly lost at 37 degrees Celsius in the absence of ligand while in the presence of 17 beta-estradiol and 4-hydroxy-tamoxifen loss in DNA-binding at elevated temperature is more gradual.
- Gene Name:
- ESR2
- Uniprot ID:
- Q92731
- Molecular Weight:
- 59215.765 Da
References
- Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M: N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006 Apr;69(8):1450-4. [16531984 ]
- Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R: Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006 Aug 15;79(12):1160-9. Epub 2006 Mar 27. [16626760 ]
- Luft S, Milki E, Glustrom E, Ampiah-Bonney R, O'Hara P. Binding of Organochloride and Pyrethroid Pesticides To Estrogen Receptors α and β: A Fluorescence Polarization Assay. Biophysical Journal 2009;96(3):444a.
- General Function:
- Zinc ion binding
- Specific Function:
- Binds to an ERR-alpha response element (ERRE) containing a single consensus half-site, 5'-TNAAGGTCA-3'. Can bind to the medium-chain acyl coenzyme A dehydrogenase (MCAD) response element NRRE-1 and may act as an important regulator of MCAD promoter. Binds to the C1 region of the lactoferrin gene promoter. Requires dimerization and the coactivator, PGC-1A, for full activity. The ERRalpha/PGC1alpha complex is a regulator of energy metabolism. Induces the expression of PERM1 in the skeletal muscle.
- Gene Name:
- ESRRA
- Uniprot ID:
- P11474
- Molecular Weight:
- 45509.11 Da
References
- Chen S, Zhou D, Yang C, Sherman M: Molecular basis for the constitutive activity of estrogen-related receptor alpha-1. J Biol Chem. 2001 Jul 27;276(30):28465-70. Epub 2001 May 16. [11356845 ]
- Chen S, Zhou D, Yang C, Okubo T, Kinoshita Y, Yu B, Kao YC, Itoh T: Modulation of aromatase expression in human breast tissue. J Steroid Biochem Mol Biol. 2001 Dec;79(1-5):35-40. [11850205 ]
- Chen S, Ye J, Kijima I, Kinoshita Y, Zhou D: Positive and negative transcriptional regulation of aromatase expression in human breast cancer tissue. J Steroid Biochem Mol Biol. 2005 May;95(1-5):17-23. [15955695 ]
- General Function:
- Serine hydrolase activity
- Specific Function:
- Terminates signal transduction at the neuromuscular junction by rapid hydrolysis of the acetylcholine released into the synaptic cleft. Role in neuronal apoptosis.
- Gene Name:
- ACHE
- Uniprot ID:
- P22303
- Molecular Weight:
- 67795.525 Da
References
- Chandra J, Durairaj G: Effect of toxaphene toxicity on enzyme activity & residue levels in vital organs of guineapig. Indian J Med Res. 1993 Aug;98:193-8. [8262581 ]
- General Function:
- Metal ion binding
- Specific Function:
- Catalyzes the formation of the signaling molecule cAMP in response to G-protein signaling. Mediates responses to increased cellular Ca(2+)/calmodulin levels (By similarity). May be involved in regulatory processes in the central nervous system. May play a role in memory and learning. Plays a role in the regulation of the circadian rhythm of daytime contrast sensitivity probably by modulating the rhythmic synthesis of cyclic AMP in the retina (By similarity).
- Gene Name:
- ADCY1
- Uniprot ID:
- Q08828
- Molecular Weight:
- 123438.85 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Manganese ion binding
- Specific Function:
- Catalyzes the formation of the signaling molecule cAMP (PubMed:12609998, PubMed:15659711, PubMed:24616449, PubMed:25040695, PubMed:24567411). May function as sensor that mediates responses to changes in cellular bicarbonate and CO(2) levels (PubMed:15659711, PubMed:17591988). Has a critical role in mammalian spermatogenesis by producing the cAMP which regulates cAMP-responsive nuclear factors indispensable for sperm maturation in the epididymis. Induces capacitation, the maturational process that sperm undergo prior to fertilization (By similarity). Involved in ciliary beat regulation (PubMed:17591988).
- Gene Name:
- ADCY10
- Uniprot ID:
- Q96PN6
- Molecular Weight:
- 187147.545 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- Catalyzes the formation of the signaling molecule cAMP in response to G-protein signaling (PubMed:15385642). Down-stream signaling cascades mediate changes in gene expression patterns and lead to increased IL6 production. Functions in signaling cascades downstream of the muscarinic acetylcholine receptors (By similarity).
- Gene Name:
- ADCY2
- Uniprot ID:
- Q08462
- Molecular Weight:
- 123602.25 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Metal ion binding
- Specific Function:
- Catalyzes the formation of the signaling molecule cAMP in response to G-protein signaling. Participates in signaling cascades triggered by odorant receptors via its function in cAMP biosynthesis. Required for the perception of odorants. Required for normal sperm motility and normal male fertility. Plays a role in regulating insulin levels and body fat accumulation in response to a high fat diet.
- Gene Name:
- ADCY3
- Uniprot ID:
- O60266
- Molecular Weight:
- 128958.905 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Metal ion binding
- Specific Function:
- Catalyzes the formation of the signaling molecule cAMP in response to G-protein signaling.
- Gene Name:
- ADCY4
- Uniprot ID:
- Q8NFM4
- Molecular Weight:
- 119792.94 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Protein heterodimerization activity
- Specific Function:
- Catalyzes the formation of the signaling molecule cAMP in response to G-protein signaling (PubMed:15385642, PubMed:26206488, PubMed:24700542). Mediates signaling downstream of ADRB1 (PubMed:24700542). Regulates the increase of free cytosolic Ca(2+) in response to increased blood glucose levels and contributes to the regulation of Ca(2+)-dependent insulin secretion (PubMed:24740569).
- Gene Name:
- ADCY5
- Uniprot ID:
- O95622
- Molecular Weight:
- 138906.37 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Protein kinase binding
- Specific Function:
- Catalyzes the formation of the signaling molecule cAMP downstream of G protein-coupled receptors (PubMed:17916776, PubMed:17110384). Functions in signaling cascades downstream of beta-adrenergic receptors in the heart and in vascular smooth muscle cells (PubMed:17916776). Functions in signaling cascades downstream of the vasopressin receptor in the kidney and has a role in renal water reabsorption. Functions in signaling cascades downstream of PTH1R and plays a role in regulating renal phosphate excretion. Functions in signaling cascades downstream of the VIP and SCT receptors in pancreas and contributes to the regulation of pancreatic amylase and fluid secretion (By similarity). Signaling mediates cAMP-dependent activation of protein kinase PKA. This promotes increased phosphorylation of various proteins, including AKT. Plays a role in regulating cardiac sarcoplasmic reticulum Ca(2+) uptake and storage, and is required for normal heart ventricular contractibility. May contribute to normal heart function (By similarity). Mediates vasodilatation after activation of beta-adrenergic receptors by isoproterenol (PubMed:17916776). Contributes to bone cell responses to mechanical stimuli (By similarity).
- Gene Name:
- ADCY6
- Uniprot ID:
- O43306
- Molecular Weight:
- 130614.095 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Metal ion binding
- Specific Function:
- This is a membrane-bound, calcium-inhibitable adenylyl cyclase.
- Gene Name:
- ADCY7
- Uniprot ID:
- P51828
- Molecular Weight:
- 120307.175 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Metal ion binding
- Specific Function:
- This is a membrane-bound, calcium-stimulable adenylyl cyclase. May be involved in learning, in memory and in drug dependence (By similarity).
- Gene Name:
- ADCY8
- Uniprot ID:
- P40145
- Molecular Weight:
- 140120.79 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Metal ion binding
- Specific Function:
- Adenylyl cyclase that catalyzes the formation of the signaling molecule cAMP in response to activation of G protein-coupled receptors (PubMed:9628827, PubMed:12972952, PubMed:15879435, PubMed:10987815). Contributes to signaling cascades activated by CRH (corticotropin-releasing factor), corticosteroids and beta-adrenergic receptors (PubMed:9628827).
- Gene Name:
- ADCY9
- Uniprot ID:
- O60503
- Molecular Weight:
- 150699.36 Da
References
- Kodavanti PR, Mehrotra BD, Chetty SC, Desaiah D: Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989 Summer;10(2):219-28. [2616064 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Steroid hormone receptors are ligand-activated transcription factors that regulate eukaryotic gene expression and affect cellular proliferation and differentiation in target tissues. Transcription factor activity is modulated by bound coactivator and corepressor proteins. Transcription activation is down-regulated by NR0B2. Activated, but not phosphorylated, by HIPK3 and ZIPK/DAPK3.
- Gene Name:
- AR
- Uniprot ID:
- P10275
- Molecular Weight:
- 98987.9 Da
References
- Schrader TJ, Cooke GM: Examination of selected food additives and organochlorine food contaminants for androgenic activity in vitro. Toxicol Sci. 2000 Feb;53(2):278-88. [10696776 ]
- General Function:
- Signal transducer activity
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of the calcium.
- Gene Name:
- ATP2C1
- Uniprot ID:
- P98194
- Molecular Weight:
- 100576.42 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium.
- Gene Name:
- ATP2C2
- Uniprot ID:
- O75185
- Molecular Weight:
- 103186.475 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA2
- Uniprot ID:
- P47869
- Molecular Weight:
- 51325.85 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA3
- Uniprot ID:
- P34903
- Molecular Weight:
- 55164.055 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA4
- Uniprot ID:
- P48169
- Molecular Weight:
- 61622.645 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Transporter activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA5
- Uniprot ID:
- P31644
- Molecular Weight:
- 52145.645 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA6
- Uniprot ID:
- Q16445
- Molecular Weight:
- 51023.69 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRB1
- Uniprot ID:
- P18505
- Molecular Weight:
- 54234.085 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB2
- Uniprot ID:
- P47870
- Molecular Weight:
- 59149.895 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-gated chloride ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB3
- Uniprot ID:
- P28472
- Molecular Weight:
- 54115.04 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRD
- Uniprot ID:
- O14764
- Molecular Weight:
- 50707.835 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRE
- Uniprot ID:
- P78334
- Molecular Weight:
- 57971.175 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG1
- Uniprot ID:
- Q8N1C3
- Molecular Weight:
- 53594.49 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRG2
- Uniprot ID:
- P18507
- Molecular Weight:
- 54161.78 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG3
- Uniprot ID:
- Q99928
- Molecular Weight:
- 54288.16 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. In the uterus, the function of the receptor appears to be related to tissue contractility. The binding of this pI subunit with other GABA(A) receptor subunits alters the sensitivity of recombinant receptors to modulatory agents such as pregnanolone.
- Gene Name:
- GABRP
- Uniprot ID:
- O00591
- Molecular Weight:
- 50639.735 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-1 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR1
- Uniprot ID:
- P24046
- Molecular Weight:
- 55882.91 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-2 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR2
- Uniprot ID:
- P28476
- Molecular Weight:
- 54150.41 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRR3
- Uniprot ID:
- A8MPY1
- Molecular Weight:
- 54271.1 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Transmembrane signaling receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRQ
- Uniprot ID:
- Q9UN88
- Molecular Weight:
- 72020.875 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Pdz domain binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B1
- Uniprot ID:
- P20020
- Molecular Weight:
- 138754.045 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Protein c-terminus binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B2
- Uniprot ID:
- Q01814
- Molecular Weight:
- 136875.18 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Pdz domain binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B3
- Uniprot ID:
- Q16720
- Molecular Weight:
- 134196.025 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Scaffold protein binding
- Specific Function:
- Calcium/calmodulin-regulated and magnesium-dependent enzyme that catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell (PubMed:8530416). By regulating sperm cell calcium homeostasis, may play a role in sperm motility (By similarity).
- Gene Name:
- ATP2B4
- Uniprot ID:
- P23634
- Molecular Weight:
- 137919.03 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Zinc ion binding
- Specific Function:
- The steroid hormones and their receptors are involved in the regulation of eukaryotic gene expression and affect cellular proliferation and differentiation in target tissues. Progesterone receptor isoform B (PRB) is involved activation of c-SRC/MAPK signaling on hormone stimulation.Isoform A: inactive in stimulating c-Src/MAPK signaling on hormone stimulation.Isoform 4: Increases mitochondrial membrane potential and cellular respiration upon stimulation by progesterone.
- Gene Name:
- PGR
- Uniprot ID:
- P06401
- Molecular Weight:
- 98979.96 Da
References
- Scippo ML, Argiris C, Van De Weerdt C, Muller M, Willemsen P, Martial J, Maghuin-Rogister G: Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal Bioanal Chem. 2004 Feb;378(3):664-9. Epub 2003 Oct 25. [14579009 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- Key regulator of striated muscle performance by acting as the major Ca(2+) ATPase responsible for the reuptake of cytosolic Ca(2+) into the sarcoplasmic reticulum. Catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A1
- Uniprot ID:
- O14983
- Molecular Weight:
- 110251.36 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- S100 protein binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Isoform 2 is involved in the regulation of the contraction/relaxation cycle.
- Gene Name:
- ATP2A2
- Uniprot ID:
- P16615
- Molecular Weight:
- 114755.765 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium. Transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A3
- Uniprot ID:
- Q93084
- Molecular Weight:
- 113976.23 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A1
- Uniprot ID:
- P05023
- Molecular Weight:
- 112895.01 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A2
- Uniprot ID:
- P50993
- Molecular Weight:
- 112264.385 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A3
- Uniprot ID:
- P13637
- Molecular Weight:
- 111747.51 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients. Plays a role in sperm motility.
- Gene Name:
- ATP1A4
- Uniprot ID:
- Q13733
- Molecular Weight:
- 114165.44 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The beta subunit regulates, through assembly of alpha/beta heterodimers, the number of sodium pumps transported to the plasma membrane.Involved in cell adhesion and establishing epithelial cell polarity.
- Gene Name:
- ATP1B1
- Uniprot ID:
- P05026
- Molecular Weight:
- 35061.07 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The exact function of the beta-2 subunit is not known.Mediates cell adhesion of neurons and astrocytes, and promotes neurite outgrowth.
- Gene Name:
- ATP1B2
- Uniprot ID:
- P14415
- Molecular Weight:
- 33366.925 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The exact function of the beta-3 subunit is not known.
- Gene Name:
- ATP1B3
- Uniprot ID:
- P54709
- Molecular Weight:
- 31512.34 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Transporter activity
- Specific Function:
- May be involved in forming the receptor site for cardiac glycoside binding or may modulate the transport function of the sodium ATPase.
- Gene Name:
- FXYD2
- Uniprot ID:
- P54710
- Molecular Weight:
- 7283.265 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.