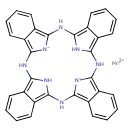
Manganese phthalocyanine (T3D1147)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-06-19 21:58:22 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:23:13 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D1147 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Manganese phthalocyanine | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Manganese phthalocyanine is a chemical compound of manganese and cyanide. Manganese is a naturally occurring metal with the symbol Mn and the atomic number 25. It does not occur naturally in its pure form, but is found in many types of rocks in combination with other substances such as oxygen, sulfur, or chlorine. Manganese occurs naturally in most foods and small amounts are needed to stay healthy, as manganese ions act as cofactors for a number of enzymes. (4, 5) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C32H22MnN8 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 573.509 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 573.135 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 14325-24-7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | manganese(2+) ion 2,11,20,29,37,38,39,40-octaazanonacyclo[28.6.1.1³,¹⁰.1¹²,¹⁹.1²¹,²⁸.0⁴,⁹.0¹³,¹⁸.0²²,²⁷.0³¹,³⁶]tetraconta-1(36),3,5,7,9,12,14,16,18,21,23,25,27,30,32,34-hexadecaene-37,39-diide | ||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | manganese(2+) ion 2,11,20,29,37,38,39,40-octaazanonacyclo[28.6.1.1³,¹⁰.1¹²,¹⁹.1²¹,²⁸.0⁴,⁹.0¹³,¹⁸.0²²,²⁷.0³¹,³⁶]tetraconta-1(36),3,5,7,9,12,14,16,18,21,23,25,27,30,32,34-hexadecaene-37,39-diide | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [Mn++].N1C2=C3C=CC=CC3=C1NC1=C3C=CC=CC3=C(NC3=C4C=CC=CC4=C(N3)NC3=C4C=CC=CC4=C(N2)[N-]3)[N-]1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C32H22N8.Mn/c1-2-10-18-17(9-1)25-33-26(18)38-28-21-13-5-6-14-22(21)30(35-28)40-32-24-16-8-7-15-23(24)31(36-32)39-29-20-12-4-3-11-19(20)27(34-29)37-25;/h1-16,33,36-40H;/q-2;+2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=LRCHRAOPRIWQNK-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as phthalocyanines. These are cyclic tetrapyrroles that contain a phthalocyanine skeleton, which consists of four isoindole-type units, with the connecting carbon atoms in the macrocycle replaced by nitrogen. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Tetrapyrroles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phthalocyanines | ||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Phthalocyanines | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral (1) ; inhalation(1) ; dermal (1) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Manganese is a cellular toxicant that can impair transport systems, enzyme activities, and receptor functions. It primarily targets the central nervous system, particularily the globus pallidus of the basal ganglia. It is believed that the manganese ion, Mn(II), enhances the autoxidation or turnover of various intracellular catecholamines, leading to increased production of free radicals, reactive oxygen species, and other cytotoxic metabolites, along with a depletion of cellular antioxidant defense mechanisms, leading to oxidative damage and selective destruction of dopaminergic neurons. In addition to dopamine, manganese is thought to perturbations other neurotransmitters, such as GABA and glutamate. In order to produce oxidative damage, manganese must first overwhelm the antioxidant enzyme manganese superoxide dismutase. The neurotoxicity of Mn(II) has also been linked to its ability to substitute for Ca(II) under physiological conditions. It can enter mitochondria via the calcium uniporter and inhibit mitochondrial oxidative phosphorylation. It may also inhibit the efflux of Ca(II), which can result in a loss of mitochondrial membrane integrity. Mn(II) has been shown to inhibit mitochondrial aconitase activity to a significant level, altering amino acid metabolism and cellular iron homeostasis. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (2, 4) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Manganese is absorbed mainly via ingestion, but can also be inhaled. It binds to alpha-2-macroglobulin, albumin, or transferrin in the plasma and is distributed to the brain and all other mammalian tissues, though it tends to accumulate more in the liver, pancreas, and kidney. Manganese is capable of existing in a number of oxidation states and is believed to undergo changes in oxidation state within the body. Manganese oxidation state can influence tissue toxicokinetic behavior, and possibly toxicity. Manganese is excreted primarily in the faeces. Cyanide is rapidly alsorbed through oral, inhalation, and dermal routes and distributed throughout the body. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. Cyanide metabolites are excreted in the urine. (1, 4) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Chronic Inhalation: 0.0003 mg/m3 (Manganese) (3) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Manganese mainly affects the nervous system and may cause behavioral changes and other nervous system effects, which include movements that may become slow and clumsy. This combination of symptoms when sufficiently severe is referred to as “manganism”. (4) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Manganese mainly affects the nervous system and may cause behavioral changes and other nervous system effects, which include movements that may become slow and clumsy. This combination of symptoms when sufficiently severe is referred to as “manganism”. (4) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Antidotes to cyanide poisoning include hydroxocobalamin and sodium nitrite, which release the cyanide from the cytochrome system, and rhodanase, which is an enzyme occurring naturally in mammals that combines serum cyanide with thiosulfate, producing comparatively harmless thiocyanate. Oxygen therapy can also be administered. (2) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 25113527 | ||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Manganese phthalocyanine | ||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D1147.pdf | ||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Iron ion binding
- Specific Function:
- Catalyzes the isomerization of citrate to isocitrate via cis-aconitate.
- Gene Name:
- ACO2
- Uniprot ID:
- Q99798
- Molecular Weight:
- 85424.745 Da
References
- Crooks DR, Ghosh MC, Braun-Sommargren M, Rouault TA, Smith DR: Manganese targets m-aconitase and activates iron regulatory protein 2 in AF5 GABAergic cells. J Neurosci Res. 2007 Jun;85(8):1797-809. [17469137 ]
- General Function:
- Metal ion binding
- Specific Function:
- Not Available
- Gene Name:
- ALPPL2
- Uniprot ID:
- P10696
- Molecular Weight:
- 57376.515 Da
References
- Gerbitz KD: Human alkaline phosphatases. II. Metalloenzyme properties of the enzyme from human liver. Hoppe Seylers Z Physiol Chem. 1977 Nov;358(11):1491-7. [924371 ]
- General Function:
- Pyrophosphatase activity
- Specific Function:
- This isozyme may play a role in skeletal mineralization.
- Gene Name:
- ALPL
- Uniprot ID:
- P05186
- Molecular Weight:
- 57304.435 Da
References
- Gerbitz KD: Human alkaline phosphatases. II. Metalloenzyme properties of the enzyme from human liver. Hoppe Seylers Z Physiol Chem. 1977 Nov;358(11):1491-7. [924371 ]
- General Function:
- Receptor binding
- Specific Function:
- Occurs in almost all aerobically respiring organisms and serves to protect cells from the toxic effects of hydrogen peroxide. Promotes growth of cells including T-cells, B-cells, myeloid leukemia cells, melanoma cells, mastocytoma cells and normal and transformed fibroblast cells.
- Gene Name:
- CAT
- Uniprot ID:
- P04040
- Molecular Weight:
- 59755.82 Da
References
- Kang YS, Lee DH, Yoon BJ, Oh DC: Purification and characterization of a catalase from photosynthetic bacterium Rhodospirillum rubrum S1 grown under anaerobic conditions. J Microbiol. 2006 Apr;44(2):185-91. [16728955 ]
- General Function:
- Iron ion binding
- Specific Function:
- Cytochrome c oxidase is the component of the respiratory chain that catalyzes the reduction of oxygen to water. Subunits 1-3 form the functional core of the enzyme complex. CO I is the catalytic subunit of the enzyme. Electrons originating in cytochrome c are transferred via the copper A center of subunit 2 and heme A of subunit 1 to the bimetallic center formed by heme A3 and copper B.
- Gene Name:
- MT-CO1
- Uniprot ID:
- P00395
- Molecular Weight:
- 57040.91 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- Cytochrome c oxidase is the component of the respiratory chain that catalyzes the reduction of oxygen to water. Subunits 1-3 form the functional core of the enzyme complex. Subunit 2 transfers the electrons from cytochrome c via its binuclear copper A center to the bimetallic center of the catalytic subunit 1.
- Gene Name:
- MT-CO2
- Uniprot ID:
- P00403
- Molecular Weight:
- 25564.73 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX4I1
- Uniprot ID:
- P13073
- Molecular Weight:
- 19576.6 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX4I2
- Uniprot ID:
- Q96KJ9
- Molecular Weight:
- 20010.02 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Metal ion binding
- Specific Function:
- This is the heme A-containing chain of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX5A
- Uniprot ID:
- P20674
- Molecular Weight:
- 16761.985 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Metal ion binding
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX5B
- Uniprot ID:
- P10606
- Molecular Weight:
- 13695.57 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6A1
- Uniprot ID:
- P12074
- Molecular Weight:
- 12154.8 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6A2
- Uniprot ID:
- Q02221
- Molecular Weight:
- 10815.32 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6C
- Uniprot ID:
- P09669
- Molecular Weight:
- 8781.36 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7A1
- Uniprot ID:
- P24310
- Molecular Weight:
- 9117.44 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7A2
- Uniprot ID:
- P14406
- Molecular Weight:
- 9395.89 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport. Plays a role in proper central nervous system (CNS) development in vertebrates.
- Gene Name:
- COX7B
- Uniprot ID:
- P24311
- Molecular Weight:
- 9160.485 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7B2
- Uniprot ID:
- Q8TF08
- Molecular Weight:
- 9077.43 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7C
- Uniprot ID:
- P15954
- Molecular Weight:
- 7245.45 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX8A
- Uniprot ID:
- P10176
- Molecular Weight:
- 7579.0 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX8C
- Uniprot ID:
- Q7Z4L0
- Molecular Weight:
- 8128.575 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Rna binding
- Specific Function:
- Iron sensor. Binds a 4Fe-4S cluster and functions as aconitase when cellular iron levels are high. Functions as mRNA binding protein that regulates uptake, sequestration and utilization of iron when cellular iron levels are low. Binds to iron-responsive elements (IRES) in target mRNA species when iron levels are low. Binding of a 4Fe-4S cluster precludes RNA binding.Catalyzes the isomerization of citrate to isocitrate via cis-aconitate.
- Gene Name:
- ACO1
- Uniprot ID:
- P21399
- Molecular Weight:
- 98398.14 Da
References
- Crooks DR, Ghosh MC, Braun-Sommargren M, Rouault TA, Smith DR: Manganese targets m-aconitase and activates iron regulatory protein 2 in AF5 GABAergic cells. J Neurosci Res. 2007 Jun;85(8):1797-809. [17469137 ]
- General Function:
- Glutathione peroxidase activity
- Specific Function:
- Protects cells and enzymes from oxidative damage, by catalyzing the reduction of hydrogen peroxide, lipid peroxides and organic hydroperoxide, by glutathione. May constitute a glutathione peroxidase-like protective system against peroxide damage in sperm membrane lipids.
- Gene Name:
- GPX5
- Uniprot ID:
- O75715
- Molecular Weight:
- 25202.14 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Protect the extracellular space from toxic effect of reactive oxygen intermediates by converting superoxide radicals into hydrogen peroxide and oxygen.
- Gene Name:
- SOD3
- Uniprot ID:
- P08294
- Molecular Weight:
- 25850.675 Da
References
- Lee WG, Hwang JH, Na BK, Cho JH, Lee HW, Cho SH, Kong Y, Song CY, Kim TS: Functional expression of a recombinant copper/zinc superoxide dismutase of filarial nematode, Brugia malayi. J Parasitol. 2005 Feb;91(1):205-8. [15856906 ]
- General Function:
- Sh3 domain binding
- Specific Function:
- Protects the hemoglobin in erythrocytes from oxidative breakdown.
- Gene Name:
- GPX1
- Uniprot ID:
- P07203
- Molecular Weight:
- 22087.94 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Glutathione peroxidase activity
- Specific Function:
- Could play a major role in protecting mammals from the toxicity of ingested organic hydroperoxides. Tert-butyl hydroperoxide, cumene hydroperoxide and linoleic acid hydroperoxide but not phosphatidycholine hydroperoxide, can act as acceptors.
- Gene Name:
- GPX2
- Uniprot ID:
- P18283
- Molecular Weight:
- 21953.835 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Transcription factor binding
- Specific Function:
- Protects cells and enzymes from oxidative damage, by catalyzing the reduction of hydrogen peroxide, lipid peroxides and organic hydroperoxide, by glutathione.
- Gene Name:
- GPX3
- Uniprot ID:
- P22352
- Molecular Weight:
- 25552.185 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Peroxidase activity
- Specific Function:
- It protects esophageal epithelia from hydrogen peroxide-induced oxidative stress. It suppresses acidic bile acid-induced reactive oxigen species (ROS) and protects against oxidative DNA damage and double-strand breaks.
- Gene Name:
- GPX7
- Uniprot ID:
- Q96SL4
- Molecular Weight:
- 20995.88 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Nadp binding
- Specific Function:
- Maintains high levels of reduced glutathione in the cytosol.
- Gene Name:
- GSR
- Uniprot ID:
- P00390
- Molecular Weight:
- 56256.565 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Translation repressor activity
- Specific Function:
- RNA-binding protein that binds to iron-responsive elements (IRES), which are stem-loop structures found in the 5'-UTR of ferritin, and delta aminolevulinic acid synthase mRNAs, and in the 3'-UTR of transferrin receptor mRNA. Binding to the IRE element in ferritin results in the repression of its mRNA translation. Binding of the protein to the transferrin receptor mRNA inhibits the degradation of this otherwise rapidly degraded mRNA.
- Gene Name:
- IREB2
- Uniprot ID:
- P48200
- Molecular Weight:
- 105043.65 Da
References
- Crooks DR, Ghosh MC, Braun-Sommargren M, Rouault TA, Smith DR: Manganese targets m-aconitase and activates iron regulatory protein 2 in AF5 GABAergic cells. J Neurosci Res. 2007 Jun;85(8):1797-809. [17469137 ]
- General Function:
- Tubulin binding
- Specific Function:
- Its primary physiological function is unclear. Has cytoprotective activity against internal or environmental stresses. May play a role in neuronal development and synaptic plasticity. May be required for neuronal myelin sheath maintenance. May play a role in iron uptake and iron homeostasis. Soluble oligomers are toxic to cultured neuroblastoma cells and induce apoptosis (in vitro) (PubMed:12732622, PubMed:19936054, PubMed:20564047). Association with GPC1 (via its heparan sulfate chains) targets PRNP to lipid rafts. Also provides Cu(2+) or ZN(2+) for the ascorbate-mediated GPC1 deaminase degradation of its heparan sulfate side chains (By similarity).
- Gene Name:
- PRNP
- Uniprot ID:
- P04156
- Molecular Weight:
- 27661.21 Da
References
- Brazier MW, Davies P, Player E, Marken F, Viles JH, Brown DR: Manganese binding to the prion protein. J Biol Chem. 2008 May 9;283(19):12831-9. doi: 10.1074/jbc.M709820200. Epub 2008 Mar 10. [18332141 ]
- General Function:
- Phospholipid-hydroperoxide glutathione peroxidase activity
- Specific Function:
- Protects cells against membrane lipid peroxidation and cell death. Required for normal sperm development and male fertility. Could play a major role in protecting mammals from the toxicity of ingested lipid hydroperoxides. Essential for embryonic development. Protects from radiation and oxidative damage.
- Gene Name:
- GPX4
- Uniprot ID:
- P36969
- Molecular Weight:
- 22174.52 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Not Available
- Specific Function:
- Not Available
- Gene Name:
- PRNT
- Uniprot ID:
- Q86SH4
- Molecular Weight:
- 10755.655 Da
References
- Brazier MW, Davies P, Player E, Marken F, Viles JH, Brown DR: Manganese binding to the prion protein. J Biol Chem. 2008 May 9;283(19):12831-9. doi: 10.1074/jbc.M709820200. Epub 2008 Mar 10. [18332141 ]
- General Function:
- Ubiquinone binding
- Specific Function:
- Membrane-anchoring subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q).
- Gene Name:
- SDHD
- Uniprot ID:
- O14521
- Molecular Weight:
- 17042.82 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Succinate dehydrogenase activity
- Specific Function:
- Flavoprotein (FP) subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q). Can act as a tumor suppressor.
- Gene Name:
- SDHA
- Uniprot ID:
- P31040
- Molecular Weight:
- 72690.975 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Ubiquinone binding
- Specific Function:
- Iron-sulfur protein (IP) subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q).
- Gene Name:
- SDHB
- Uniprot ID:
- P21912
- Molecular Weight:
- 31629.365 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Succinate dehydrogenase activity
- Specific Function:
- Membrane-anchoring subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q).
- Gene Name:
- SDHC
- Uniprot ID:
- Q99643
- Molecular Weight:
- 18610.03 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Destroys radicals which are normally produced within the cells and which are toxic to biological systems.
- Gene Name:
- SOD1
- Uniprot ID:
- P00441
- Molecular Weight:
- 15935.685 Da
References
- Lee WG, Hwang JH, Na BK, Cho JH, Lee HW, Cho SH, Kong Y, Song CY, Kim TS: Functional expression of a recombinant copper/zinc superoxide dismutase of filarial nematode, Brugia malayi. J Parasitol. 2005 Feb;91(1):205-8. [15856906 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- This is a copper-containing oxidase that functions in the formation of pigments such as melanins and other polyphenolic compounds. Catalyzes the rate-limiting conversions of tyrosine to DOPA, DOPA to DOPA-quinone and possibly 5,6-dihydroxyindole to indole-5,6 quinone.
- Gene Name:
- TYR
- Uniprot ID:
- P14679
- Molecular Weight:
- 60392.69 Da
References
- Laufer Z, Beckett RP, Minibayeva FV: Co-occurrence of the multicopper oxidases tyrosinase and laccase in lichens in sub-order peltigerineae. Ann Bot. 2006 Nov;98(5):1035-42. Epub 2006 Sep 1. [16950829 ]