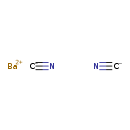Barium cyanide (T3D1103)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-06-19 21:58:18 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:23:09 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D1103 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Barium cyanide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Barium cyanide is a chemical compound of barium and cyanide. Barium is a metallic alkaline earth metal with the symbol Ba, and atomic number 56. It never occurs in nature in its pure form due to its reactivity with air, but combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds that may be found as minerals. (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C2BaN2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 189.362 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 189.911 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 542-62-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | barium(2+) ion bis(iminomethanide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | barium(2+) ion bis(cyanide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [Ba++].[C-]#N.[C-]#N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/2CN.Ba/c2*1-2;/q2*-1;+2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=UNLSXXHOHZUADN-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as alkaline earth metal nitrides. These are inorganic compounds of nitrogen where nitrogen has a formal oxidation state of -3, and the heaviest metal atom is an alkaline earth metal. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Mixed metal/non-metal compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Alkaline earth metal organides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Alkaline earth metal nitrides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Alkaline earth metal nitrides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral (2) ; inhalation (2) ; dermal (2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Barium is a competitive potassium channel antagonist that blocks the passive efflux of intracellular potassium, resulting in a shift of potassium from extracellular to intracellular compartments. The intracellular translocation of potassium results in a decreased resting membrane potential, making the muscle fibers electrically unexcitable and causing paralysis. Some of these barium's effects may also be due to barium induced neuromuscular blockade and membrane depolarization. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (3, 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Barium compounds are absorbed via ingestion and inhalation, the extent of which depends on the individual compound. In the body, the majority of the barium is found in the bone, while small amounts exists in the muscle, adipose, skin, and connective tissue. Barium is not metabolized in the body, but it may be transported or incorporated into complexes or tissues. Barium is excreted in the urine and faeces. Cyanide is rapidly alsorbed through oral, inhalation, and dermal routes and distributed throughout the body. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. Cyanide metabolites are excreted in the urine. (2, 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | 1 to 15 grams for an adult human (barium salts). (1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Intermediate Oral: 0.2 mg/kg/day (Barium) (4) Chronic Oral: 0.2 mg/kg/day (Barium) (4) Acute Inhalation: 0.00003 mg/m3 (Cadmium) (4) Chronic Inhalation: 0.00001 mg/m3 (Cadmium) (4) Intermediate Oral: 0.0005 mg/kg/day (Cadmium) (4) Chronic Oral: 0.0001 mg/kg/day (Cadmium) (4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | The health effects of the different barium compounds depend on how well the compound dissolves in water or the stomach contents. At low doses, barium acts as a muscle stimulant, while higher doses affect the nervous system, causing cardiac irregularities, tremors, weakness, anxiety, dyspnea, paralysisand possibly death. Barium may also cause gastrointestinal disturbances, damage the kidneys and cause decreases in body weight. Exposure to high levels of cyanide for a short time harms the brain and heart and can even cause coma, seizures, apnea, cardiac arrest and death. Chronic inhalation of cyanide causes breathing difficulties, chest pain, vomiting, blood changes, headaches, and enlargement of the thyroid gland. Skin contact with cyanide salts can irritate and produce sores. (2, 3, 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Ingesting excess barium may cause vomiting, abdominal cramps, diarrhea, difficulties in breathing, increased or decreased blood pressure, numbness around the face, and muscle weakness. High levels may result in changes in heart rhythm or paralysis and possibly death. Cyanide poisoning is identified by rapid, deep breathing and shortness of breath, general weakness, giddiness, headaches, vertigo, confusion, convulsions/seizures and eventually loss of consciousness. (2, 3, 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Intravenous infusion of potassium often relieves many of the symptoms of barium toxicity. Antidotes to cyanide poisoning include hydroxocobalamin and sodium nitrite, which release the cyanide from the cytochrome system, and rhodanase, which is an enzyme occurring naturally in mammals that combines serum cyanide with thiosulfate, producing comparatively harmless thiocyanate. Oxygen therapy can also be administered. (3, 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 6093184 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Barium cyanide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | 6374 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D1103.pdf | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Succinate dehydrogenase activity
- Specific Function:
- Flavoprotein (FP) subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q). Can act as a tumor suppressor.
- Gene Name:
- SDHA
- Uniprot ID:
- P31040
- Molecular Weight:
- 72690.975 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- Bolognesi M, Rosano C, Losso R, Borassi A, Rizzi M, Wittenberg JB, Boffi A, Ascenzi P: Cyanide binding to Lucina pectinata hemoglobin I and to sperm whale myoglobin: an x-ray crystallographic study. Biophys J. 1999 Aug;77(2):1093-9. [10423453 ]
- General Function:
- Ubiquinone binding
- Specific Function:
- Iron-sulfur protein (IP) subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q).
- Gene Name:
- SDHB
- Uniprot ID:
- P21912
- Molecular Weight:
- 31629.365 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- Bolognesi M, Rosano C, Losso R, Borassi A, Rizzi M, Wittenberg JB, Boffi A, Ascenzi P: Cyanide binding to Lucina pectinata hemoglobin I and to sperm whale myoglobin: an x-ray crystallographic study. Biophys J. 1999 Aug;77(2):1093-9. [10423453 ]
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Gene Name:
- KCNJ1
- Uniprot ID:
- P48048
- Molecular Weight:
- 44794.6 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- This receptor is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by extracellular barium (By similarity). Subunit of ATP-sensitive potassium channels (KATP). Can form cardiac and smooth muscle-type KATP channels with ABCC9. KCNJ11 forms the channel pore while ABCC9 is required for activation and regulation.
- Gene Name:
- KCNJ11
- Uniprot ID:
- Q14654
- Molecular Weight:
- 43540.375 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- Inward rectifying potassium channel that is activated by phosphatidylinositol 4,5-bisphosphate and that probably participates in controlling the resting membrane potential in electrically excitable cells. Probably participates in establishing action potential waveform and excitability of neuronal and muscle tissues. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium.
- Gene Name:
- KCNJ12
- Uniprot ID:
- Q14500
- Molecular Weight:
- 49000.6 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- This potassium channel is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by external barium (By similarity).
- Gene Name:
- KCNJ8
- Uniprot ID:
- Q15842
- Molecular Weight:
- 47967.455 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
7. ATP-sensitive potassium channel (Protein Group)
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Included Proteins:
- P48048 , P78508 , Q14654 , Q14500 , Q9UNX9 , Q99712 , Q15842
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
8. ATP-sensitive potassium channel (Protein Group)
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Included Proteins:
- P48048 , P78508 , Q14654 , Q14500 , Q9UNX9 , Q99712 , Q15842
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
9. ATP-sensitive potassium channel (Protein Group)
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Included Proteins:
- P48048 , P78508 , Q14654 , Q14500 , Q9UNX9 , Q99712 , Q15842
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Metal ion binding
- Specific Function:
- Not Available
- Gene Name:
- ALPPL2
- Uniprot ID:
- P10696
- Molecular Weight:
- 57376.515 Da
References
- Gerbitz KD: Human alkaline phosphatases. II. Metalloenzyme properties of the enzyme from human liver. Hoppe Seylers Z Physiol Chem. 1977 Nov;358(11):1491-7. [924371 ]
- General Function:
- Pyrophosphatase activity
- Specific Function:
- This isozyme may play a role in skeletal mineralization.
- Gene Name:
- ALPL
- Uniprot ID:
- P05186
- Molecular Weight:
- 57304.435 Da
References
- Gerbitz KD: Human alkaline phosphatases. II. Metalloenzyme properties of the enzyme from human liver. Hoppe Seylers Z Physiol Chem. 1977 Nov;358(11):1491-7. [924371 ]
- General Function:
- Titin binding
- Specific Function:
- Calmodulin mediates the control of a large number of enzymes, ion channels, aquaporins and other proteins by Ca(2+). Among the enzymes to be stimulated by the calmodulin-Ca(2+) complex are a number of protein kinases and phosphatases. Together with CCP110 and centrin, is involved in a genetic pathway that regulates the centrosome cycle and progression through cytokinesis.
- Gene Name:
- CALM1
- Uniprot ID:
- P0DP23
- Molecular Weight:
- 16837.47 Da
References
- Kursula P, Majava V: A structural insight into lead neurotoxicity and calmodulin activation by heavy metals. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 Aug 1;63(Pt 8):653-6. Epub 2007 Jul 28. [17671360 ]
- General Function:
- Receptor binding
- Specific Function:
- Occurs in almost all aerobically respiring organisms and serves to protect cells from the toxic effects of hydrogen peroxide. Promotes growth of cells including T-cells, B-cells, myeloid leukemia cells, melanoma cells, mastocytoma cells and normal and transformed fibroblast cells.
- Gene Name:
- CAT
- Uniprot ID:
- P04040
- Molecular Weight:
- 59755.82 Da
References
- Kang YS, Lee DH, Yoon BJ, Oh DC: Purification and characterization of a catalase from photosynthetic bacterium Rhodospirillum rubrum S1 grown under anaerobic conditions. J Microbiol. 2006 Apr;44(2):185-91. [16728955 ]
- General Function:
- Iron ion binding
- Specific Function:
- Cytochrome c oxidase is the component of the respiratory chain that catalyzes the reduction of oxygen to water. Subunits 1-3 form the functional core of the enzyme complex. CO I is the catalytic subunit of the enzyme. Electrons originating in cytochrome c are transferred via the copper A center of subunit 2 and heme A of subunit 1 to the bimetallic center formed by heme A3 and copper B.
- Gene Name:
- MT-CO1
- Uniprot ID:
- P00395
- Molecular Weight:
- 57040.91 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- Cytochrome c oxidase is the component of the respiratory chain that catalyzes the reduction of oxygen to water. Subunits 1-3 form the functional core of the enzyme complex. Subunit 2 transfers the electrons from cytochrome c via its binuclear copper A center to the bimetallic center of the catalytic subunit 1.
- Gene Name:
- MT-CO2
- Uniprot ID:
- P00403
- Molecular Weight:
- 25564.73 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX4I1
- Uniprot ID:
- P13073
- Molecular Weight:
- 19576.6 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX4I2
- Uniprot ID:
- Q96KJ9
- Molecular Weight:
- 20010.02 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Metal ion binding
- Specific Function:
- This is the heme A-containing chain of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX5A
- Uniprot ID:
- P20674
- Molecular Weight:
- 16761.985 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Metal ion binding
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX5B
- Uniprot ID:
- P10606
- Molecular Weight:
- 13695.57 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6A1
- Uniprot ID:
- P12074
- Molecular Weight:
- 12154.8 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6A2
- Uniprot ID:
- Q02221
- Molecular Weight:
- 10815.32 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX6C
- Uniprot ID:
- P09669
- Molecular Weight:
- 8781.36 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7A1
- Uniprot ID:
- P24310
- Molecular Weight:
- 9117.44 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7A2
- Uniprot ID:
- P14406
- Molecular Weight:
- 9395.89 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport. Plays a role in proper central nervous system (CNS) development in vertebrates.
- Gene Name:
- COX7B
- Uniprot ID:
- P24311
- Molecular Weight:
- 9160.485 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7B2
- Uniprot ID:
- Q8TF08
- Molecular Weight:
- 9077.43 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX7C
- Uniprot ID:
- P15954
- Molecular Weight:
- 7245.45 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX8A
- Uniprot ID:
- P10176
- Molecular Weight:
- 7579.0 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Cytochrome-c oxidase activity
- Specific Function:
- This protein is one of the nuclear-coded polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport.
- Gene Name:
- COX8C
- Uniprot ID:
- Q7Z4L0
- Molecular Weight:
- 8128.575 Da
References
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
- General Function:
- Glutathione peroxidase activity
- Specific Function:
- Protects cells and enzymes from oxidative damage, by catalyzing the reduction of hydrogen peroxide, lipid peroxides and organic hydroperoxide, by glutathione. May constitute a glutathione peroxidase-like protective system against peroxide damage in sperm membrane lipids.
- Gene Name:
- GPX5
- Uniprot ID:
- O75715
- Molecular Weight:
- 25202.14 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Protect the extracellular space from toxic effect of reactive oxygen intermediates by converting superoxide radicals into hydrogen peroxide and oxygen.
- Gene Name:
- SOD3
- Uniprot ID:
- P08294
- Molecular Weight:
- 25850.675 Da
References
- Lee WG, Hwang JH, Na BK, Cho JH, Lee HW, Cho SH, Kong Y, Song CY, Kim TS: Functional expression of a recombinant copper/zinc superoxide dismutase of filarial nematode, Brugia malayi. J Parasitol. 2005 Feb;91(1):205-8. [15856906 ]
- General Function:
- G-protein activated inward rectifier potassium channel activity
- Specific Function:
- This potassium channel is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This receptor plays a crucial role in regulating the heartbeat.
- Gene Name:
- KCNJ3
- Uniprot ID:
- P48549
- Molecular Weight:
- 56602.84 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- This potassium channel may be involved in the regulation of insulin secretion by glucose and/or neurotransmitters acting through G-protein-coupled receptors. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium.
- Gene Name:
- KCNJ6
- Uniprot ID:
- P48051
- Molecular Weight:
- 48450.96 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- G-protein activated inward rectifier potassium channel activity
- Specific Function:
- This receptor is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium (By similarity).
- Gene Name:
- KCNJ9
- Uniprot ID:
- Q92806
- Molecular Weight:
- 44019.45 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- G-protein activated inward rectifier potassium channel activity
- Specific Function:
- This potassium channel is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by external barium.
- Gene Name:
- KCNJ5
- Uniprot ID:
- P48544
- Molecular Weight:
- 47667.3 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Sh3 domain binding
- Specific Function:
- Protects the hemoglobin in erythrocytes from oxidative breakdown.
- Gene Name:
- GPX1
- Uniprot ID:
- P07203
- Molecular Weight:
- 22087.94 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Glutathione peroxidase activity
- Specific Function:
- Could play a major role in protecting mammals from the toxicity of ingested organic hydroperoxides. Tert-butyl hydroperoxide, cumene hydroperoxide and linoleic acid hydroperoxide but not phosphatidycholine hydroperoxide, can act as acceptors.
- Gene Name:
- GPX2
- Uniprot ID:
- P18283
- Molecular Weight:
- 21953.835 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Transcription factor binding
- Specific Function:
- Protects cells and enzymes from oxidative damage, by catalyzing the reduction of hydrogen peroxide, lipid peroxides and organic hydroperoxide, by glutathione.
- Gene Name:
- GPX3
- Uniprot ID:
- P22352
- Molecular Weight:
- 25552.185 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Peroxidase activity
- Specific Function:
- It protects esophageal epithelia from hydrogen peroxide-induced oxidative stress. It suppresses acidic bile acid-induced reactive oxigen species (ROS) and protects against oxidative DNA damage and double-strand breaks.
- Gene Name:
- GPX7
- Uniprot ID:
- Q96SL4
- Molecular Weight:
- 20995.88 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Nadp binding
- Specific Function:
- Maintains high levels of reduced glutathione in the cytosol.
- Gene Name:
- GSR
- Uniprot ID:
- P00390
- Molecular Weight:
- 56256.565 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Oxygen transporter activity
- Specific Function:
- Involved in oxygen transport from the lung to the various peripheral tissues.
- Gene Name:
- HBA1
- Uniprot ID:
- P69905
- Molecular Weight:
- 15257.405 Da
References
- Bolognesi M, Rosano C, Losso R, Borassi A, Rizzi M, Wittenberg JB, Boffi A, Ascenzi P: Cyanide binding to Lucina pectinata hemoglobin I and to sperm whale myoglobin: an x-ray crystallographic study. Biophys J. 1999 Aug;77(2):1093-9. [10423453 ]
- General Function:
- Oxygen transporter activity
- Specific Function:
- Involved in oxygen transport from the lung to the various peripheral tissues.LVV-hemorphin-7 potentiates the activity of bradykinin, causing a decrease in blood pressure.Spinorphin: functions as an endogenous inhibitor of enkephalin-degrading enzymes such as DPP3, and as a selective antagonist of the P2RX3 receptor which is involved in pain signaling, these properties implicate it as a regulator of pain and inflammation.
- Gene Name:
- HBB
- Uniprot ID:
- P68871
- Molecular Weight:
- 15998.34 Da
References
- Bolognesi M, Rosano C, Losso R, Borassi A, Rizzi M, Wittenberg JB, Boffi A, Ascenzi P: Cyanide binding to Lucina pectinata hemoglobin I and to sperm whale myoglobin: an x-ray crystallographic study. Biophys J. 1999 Aug;77(2):1093-9. [10423453 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. KCNJ13 has a very low single channel conductance, low sensitivity to block by external barium and cesium, and no dependence of its inward rectification properties on the internal blocking particle magnesium.
- Gene Name:
- KCNJ13
- Uniprot ID:
- O60928
- Molecular Weight:
- 40529.195 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. KCNJ16 may be involved in the regulation of fluid and pH balance.
- Gene Name:
- KCNJ16
- Uniprot ID:
- Q9NPI9
- Molecular Weight:
- 47948.585 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Voltage-gated potassium channel activity involved in cardiac muscle cell action potential repolarization
- Specific Function:
- Probably participates in establishing action potential waveform and excitability of neuronal and muscle tissues. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by extracellular barium or cesium.
- Gene Name:
- KCNJ2
- Uniprot ID:
- P63252
- Molecular Weight:
- 48287.82 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Pdz domain binding
- Specific Function:
- Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by extracellular barium and cesium (By similarity).
- Gene Name:
- KCNJ4
- Uniprot ID:
- P48050
- Molecular Weight:
- 49499.61 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Phospholipid-hydroperoxide glutathione peroxidase activity
- Specific Function:
- Protects cells against membrane lipid peroxidation and cell death. Required for normal sperm development and male fertility. Could play a major role in protecting mammals from the toxicity of ingested lipid hydroperoxides. Essential for embryonic development. Protects from radiation and oxidative damage.
- Gene Name:
- GPX4
- Uniprot ID:
- P36969
- Molecular Weight:
- 22174.52 Da
References
- Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1116-22. [7437059 ]
- General Function:
- Voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization
- Specific Function:
- Potassium channel that plays an important role in a number of tissues, including heart, inner ear, stomach and colon (By similarity) (PubMed:10646604). Associates with KCNE beta subunits that modulates current kinetics (By similarity) (PubMed:9312006, PubMed:9108097, PubMed:8900283, PubMed:10646604, PubMed:11101505, PubMed:19687231). Induces a voltage-dependent by rapidly activating and slowly deactivating potassium-selective outward current (By similarity) (PubMed:9312006, PubMed:9108097, PubMed:8900283, PubMed:10646604, PubMed:11101505). Promotes also a delayed voltage activated potassium current showing outward rectification characteristic (By similarity). During beta-adrenergic receptor stimulation participates in cardiac repolarization by associating with KCNE1 to form the I(Ks) cardiac potassium current that increases the amplitude and slows down the activation kinetics of outward potassium current I(Ks) (By similarity) (PubMed:9312006, PubMed:9108097, PubMed:8900283, PubMed:10646604, PubMed:11101505). Muscarinic agonist oxotremorine-M strongly suppresses KCNQ1/KCNE1 current (PubMed:10713961). When associated with KCNE3, forms the potassium channel that is important for cyclic AMP-stimulated intestinal secretion of chloride ions (PubMed:10646604). This interaction with KCNE3 is reduced by 17beta-estradiol, resulting in the reduction of currents (By similarity). During conditions of increased substrate load, maintains the driving force for proximal tubular and intestinal sodium ions absorption, gastric acid secretion, and cAMP-induced jejunal chloride ions secretion (By similarity). Allows the provision of potassium ions to the luminal membrane of the secretory canaliculus in the resting state as well as during stimulated acid secretion (By similarity). When associated with KCNE2, forms an heterooligomer complex leading to currents with an apparently instantaneous activation, a rapid deactivation process and a linear current-voltage relationship and decreases the amplitude of the outward current (PubMed:11101505). When associated with KCNE4, inhibits voltage-gated potassium channel activity (PubMed:19687231). When associated with KCNE5, this complex only conducts current upon strong and continued depolarization (PubMed:12324418). Also forms an heterotetramer with KCNQ5; has a voltage-gated potassium channel activity (PubMed:24855057). Binds with phosphatidylinositol 4,5-bisphosphate (PubMed:25037568).Isoform 2: Non-functional alone but modulatory when coexpressed with the full-length isoform 1.
- Gene Name:
- KCNQ1
- Uniprot ID:
- P51787
- Molecular Weight:
- 74697.925 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. Associates with KCNQ3 to form a potassium channel with essentially identical properties to the channel underlying the native M-current, a slowly activating and deactivating potassium conductance which plays a critical role in determining the subthreshold electrical excitability of neurons as well as the responsiveness to synaptic inputs. KCNQ2/KCNQ3 current is blocked by linopirdine and XE991, and activated by the anticonvulsant retigabine. Muscarinic agonist oxotremorine-M strongly suppress KCNQ2/KCNQ3 current in cells in which cloned KCNQ2/KCNQ3 channels were coexpressed with M1 muscarinic receptors.
- Gene Name:
- KCNQ2
- Uniprot ID:
- O43526
- Molecular Weight:
- 95846.575 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. Associates with KCNQ2 or KCNQ5 to form a potassium channel with essentially identical properties to the channel underlying the native M-current, a slowly activating and deactivating potassium conductance which plays a critical role in determining the subthreshold electrical excitability of neurons as well as the responsiveness to synaptic inputs.
- Gene Name:
- KCNQ3
- Uniprot ID:
- O43525
- Molecular Weight:
- 96741.515 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. May underlie a potassium current involved in regulating the excitability of sensory cells of the cochlea. KCNQ4 channels are blocked by linopirdin, XE991 and bepridil, whereas clofilium is without significant effect. Muscarinic agonist oxotremorine-M strongly suppress KCNQ4 current in CHO cells in which cloned KCNQ4 channels were coexpressed with M1 muscarinic receptors.
- Gene Name:
- KCNQ4
- Uniprot ID:
- P56696
- Molecular Weight:
- 77099.99 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. Associates with KCNQ3 to form a potassium channel which contributes to M-type current, a slowly activating and deactivating potassium conductance which plays a critical role in determining the subthreshold electrical excitability of neurons. May contribute, with other potassium channels, to the molecular diversity of a heterogeneous population of M-channels, varying in kinetic and pharmacological properties, which underlie this physiologically important current. Insensitive to tetraethylammonium, but inhibited by barium, linopirdine and XE991. Activated by niflumic acid and the anticonvulsant retigabine. Muscarine suppresses KCNQ5 current in Xenopus oocytes in which cloned KCNQ5 channels were coexpressed with M(1) muscarinic receptors.
- Gene Name:
- KCNQ5
- Uniprot ID:
- Q9NR82
- Molecular Weight:
- 102178.015 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Ubiquinone binding
- Specific Function:
- Membrane-anchoring subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q).
- Gene Name:
- SDHD
- Uniprot ID:
- O14521
- Molecular Weight:
- 17042.82 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Succinate dehydrogenase activity
- Specific Function:
- Membrane-anchoring subunit of succinate dehydrogenase (SDH) that is involved in complex II of the mitochondrial electron transport chain and is responsible for transferring electrons from succinate to ubiquinone (coenzyme Q).
- Gene Name:
- SDHC
- Uniprot ID:
- Q99643
- Molecular Weight:
- 18610.03 Da
References
- Ardelt BK, Borowitz JL, Isom GE: Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989 Jun 1;56(2):147-54. [2734799 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Destroys radicals which are normally produced within the cells and which are toxic to biological systems.
- Gene Name:
- SOD1
- Uniprot ID:
- P00441
- Molecular Weight:
- 15935.685 Da
References
- Lee WG, Hwang JH, Na BK, Cho JH, Lee HW, Cho SH, Kong Y, Song CY, Kim TS: Functional expression of a recombinant copper/zinc superoxide dismutase of filarial nematode, Brugia malayi. J Parasitol. 2005 Feb;91(1):205-8. [15856906 ]
- General Function:
- Protein homodimerization activity
- Specific Function:
- This is a copper-containing oxidase that functions in the formation of pigments such as melanins and other polyphenolic compounds. Catalyzes the rate-limiting conversions of tyrosine to DOPA, DOPA to DOPA-quinone and possibly 5,6-dihydroxyindole to indole-5,6 quinone.
- Gene Name:
- TYR
- Uniprot ID:
- P14679
- Molecular Weight:
- 60392.69 Da
References
- Laufer Z, Beckett RP, Minibayeva FV: Co-occurrence of the multicopper oxidases tyrosinase and laccase in lichens in sub-order peltigerineae. Ann Bot. 2006 Nov;98(5):1035-42. Epub 2006 Sep 1. [16950829 ]
- General Function:
- Xanthine oxidase activity
- Specific Function:
- Key enzyme in purine degradation. Catalyzes the oxidation of hypoxanthine to xanthine. Catalyzes the oxidation of xanthine to uric acid. Contributes to the generation of reactive oxygen species. Has also low oxidase activity towards aldehydes (in vitro).
- Gene Name:
- XDH
- Uniprot ID:
- P47989
- Molecular Weight:
- 146422.99 Da
References
- Bolognesi M, Rosano C, Losso R, Borassi A, Rizzi M, Wittenberg JB, Boffi A, Ascenzi P: Cyanide binding to Lucina pectinata hemoglobin I and to sperm whale myoglobin: an x-ray crystallographic study. Biophys J. 1999 Aug;77(2):1093-9. [10423453 ]